Korean black ginseng extract alleviates Alzheimer's disease-related cognitive impairment by activating the Nrf2/HO-1 pathway and suppressing the p38 MAPK/NF-κB/STAT3 pathways and NLRP3 inflammasome via TLR2 and TLR4 modulation
- PMID: 40453349
- PMCID: PMC12125584
- DOI: 10.1016/j.jgr.2025.02.002
Korean black ginseng extract alleviates Alzheimer's disease-related cognitive impairment by activating the Nrf2/HO-1 pathway and suppressing the p38 MAPK/NF-κB/STAT3 pathways and NLRP3 inflammasome via TLR2 and TLR4 modulation
Abstract
Background: Korean black ginseng, a specially processed ginseng through repeat steaming and drying, has various pharmacological effects. However, its role i n cognitive impairment remains unclear.
Purpose and methods: This study examined whether Korean black ginseng extract (BGE; 50 and 100 mg/kg, orally, 18 weeks) may mitigate cognitive impairment in a 5xFAD mouse model of Alzheimer's disease (AD).
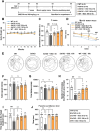
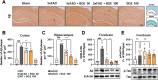
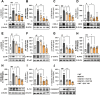
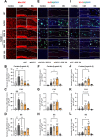
Results: BGE significantly improved cognitive performance in 5xFAD mice, associated with reduced Aβ accumulation in the frontal cortex and hippocampus. BGE suppressed microglial and astrocytic activation, alongside the downregulation of pro-inflammatory cytokines (interleukin-6 and tumor necrosis factor-α) and enzymes (cyclooxygenase-2 and inducible nitric oxide synthase). These changes coincided with the inhibition of key inflammatory signaling pathways, such as p38 mitogen-activated protein kinase (MAPK), nuclear factor kappa B (NF-κB)/p65, signal transducer and activator of transcription (STAT) 3, and NOD-like receptor protein 3 (NLRP3) inflammasome. Furthermore, BGE reduced the generation of reactive oxygen species and enhanced the nuclear-E2-related factor 2 (Nrf2)-heme oxygenase 1 (HO-1) signaling pathway in the brains linked to the downregulation of toll-like receptors (TLR)-2 and TLR-4 in the brain.
Conclusion: Taken together, BGE could improve AD-related cognitive decline and neurodegeneration by simultaneously regulating anti-inflammatory pathways (p38 MAPK/NF-κB/STAT3 and NLRP3 inflammasome) and an antioxidant pathway (Nrf2/HO-1) via modulation of TLR2/4.
Keywords: Alzheimer's disease; Anti-inflammation; Anti-oxidation; Korean black ginseng; Toll-like receptor 2/4.
© 2025 The Korean Society of Ginseng. Publishing services by Elsevier B.V.
Conflict of interest statement
None of the authors have declared any conflicts of interest related to this subject.
Figures



References
-
- Ren D., Fu Y., Wang L., Liu J., Zhong X., Yuan J., et al. Tetrandrine ameliorated Alzheimer's disease through suppressing microglial inflammatory activation and neurotoxicity in the 5XFAD mouse. Phytomedicine. 2021;90 - PubMed
-
- Leng F., Edison P. Neuroinflammation and microglial activation in Alzheimer disease: where do we go from here? Nat Rev Neurol. 2021;17:157–172. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

