The striatal compartments, striosome and matrix, are embedded in largely distinct resting-state functional networks
- PMID: 40453419
- PMCID: PMC12122536
- DOI: 10.3389/fncir.2025.1514937
The striatal compartments, striosome and matrix, are embedded in largely distinct resting-state functional networks
Abstract
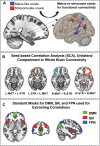
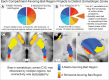
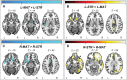
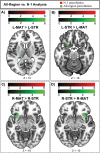
The striatum is divided into two interdigitated tissue compartments, the striosome and matrix. These compartments exhibit distinct anatomical, neurochemical, and pharmacological characteristics and have separable roles in motor and mood functions. Little is known about the functions of these compartments in humans. While compartment-specific roles in neuropsychiatric diseases have been hypothesized, they have yet to be directly tested. Investigating compartment-specific functions is crucial for understanding the symptoms produced by striatal injury, and to elucidating the roles of each compartment in healthy human skills and behaviors. We mapped the functional networks of striosome-like and matrix-like voxels in humans in-vivo. We utilized a diverse cohort of 674 healthy adults, derived from the Human Connectome Project, including all subjects with complete diffusion and functional MRI data and excluding subjects with substance use disorders. We identified striatal voxels with striosome-like and matrix-like structural connectivity using probabilistic diffusion tractography. We then investigated resting-state functional connectivity (rsFC) using these compartment-like voxels as seeds. We found widespread differences in rsFC between striosome-like and matrix-like seeds (p < 0.05, family wise error corrected for multiple comparisons), suggesting that striosome and matrix occupy distinct functional networks. Slightly shifting seed voxel locations (<4 mm) eliminated these rsFC differences, underscoring the anatomic precision of these networks. Striosome-seeded networks exhibited ipsilateral dominance; matrix-seeded networks had contralateral dominance. Next, we assessed compartment-specific engagement with the triple-network model (default mode, salience, and frontoparietal networks). Striosome-like voxels dominated rsFC with the default mode network bilaterally. The anterior insula (a primary node in the salience network) had higher rsFC with striosome-like voxels. The inferior and middle frontal cortices (primary nodes, frontoparietal network) had stronger rsFC with matrix-like voxels on the left, and striosome-like voxels on the right. Since striosome-like and matrix-like voxels occupy highly segregated rsFC networks, striosome-selective injury may produce different motor, cognitive, and behavioral symptoms than matrix-selective injury. Moreover, compartment-specific rsFC abnormalities may be identifiable before disease-related structural injuries are evident. Localizing rsFC differences provides an anatomic substrate for understanding how the tissue-level organization of the striatum underpins complex brain networks, and how compartment-specific injury may contribute to the symptoms of specific neuropsychiatric disorders.
Keywords: compartment; diffusion tractography; functional MRI; functional connectivity; matrix; striatum; striosome; structural connectivity.
Copyright © 2025 Sadiq, Funk and Waugh.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





Update of
-
The striatal compartments, striosome and matrix, are embedded in largely distinct resting state functional networks.bioRxiv [Preprint]. 2024 Dec 17:2024.12.13.628392. doi: 10.1101/2024.12.13.628392. bioRxiv. 2024. Update in: Front Neural Circuits. 2025 May 16;19:1514937. doi: 10.3389/fncir.2025.1514937. PMID: 39763746 Free PMC article. Updated. Preprint.
References
-
- Barto A. G. (1995). “Adaptive critics and the basal ganglia,” in Models of Information Processing in the Basal, eds Ganglia J. C., Houk J., Beiser D. (Cambridge, MA: MIT Press; ).
-
- Benjamini Y., Hochberg Y. (1995). Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B 57 289–300. 10.1111/j.2517-6161.1995.tb02031.x - DOI
-
- Berendse H., Voorn P., te Kortschot A., Groenewegen H. J. (1988). Nuclear origin of thalamic afferents of the ventral striatum determines their relation to patch/matrix configurations in enkephalin-immunoreactivity in the rat. J. Chem. Neuroanat. 1 3–10. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

