Immune characterization of lupus nephritis patients undergoing dialysis
- PMID: 40453547
- PMCID: PMC12124682
- DOI: 10.1016/j.jtauto.2025.100290
Immune characterization of lupus nephritis patients undergoing dialysis
Abstract
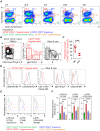
Systemic lupus erythematosus (SLE) activity decreases in some patients with end-stage kidney disease (ESKD). The impact of ESKD on the immune cell profile of SLE patients and lupus activity remains unclear. In this study, we aimed at characterizing immunologically inactive and active SLE patients undergoing dialysis therapy. Based on multi-parametric flow cytometry assays, an extensive immunophenotyping was performed on blood samples from 47 SLE patients undergoing hemodialysis, 10 non-dialyzed SLE patients with active lupus nephritis (aLN), 6 non-dialyzed patients with a history of LN currently in remission (rLN), and 20 healthy volunteers (HV) as controls (ClinicalTrials.gov Identifier: NCT03921398). The hemodialysis group was composed of 16 SLE patients with inactive disease (iHD), 22 with sustained low disease activity with a non-renal SLEDAI ≤4 (aHD≤4), and 9 highly active SLE patients (aHD>4). A factorial discriminant analysis was performed to validate the association between immune cell signatures and lupus activity. By compiling 12 cellular variables, we describe immune profiles related to highly active SLE patients or associated with both inactive and low-disease activity groups. As non-dialyzed active SLE patients, active patients undergoing hemodialysis showed a specific combination of increased numbers of circulating CD19hi CD27- "atypical naive" B cells, plasmablasts, CD16+ inflammatory monocytes and a basopenia. This study brings a comprehensive overview of immune cell signatures observed in SLE patients undergoing dialysis. We propose a simple immunophenotypic approach for the assessment of lupus activity that may provide help to data-driven personalized medicine in hemodialyzed SLE patients.
Keywords: Dialysis; Disease activity; End stage kidney disease; Immunophenotyping; Lupus nephritis; Systemic lupus erythematosus.
© 2025 The Authors.
Conflict of interest statement
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:Eric Daugas reports financial support was provided by 10.13039/501100001665French National Research Agency. Nicolas CHARLES reports financial support was provided by 10.13039/501100001665French National Research Agency. Nicolas CHARLES reports financial support was provided by 10.13039/501100002915Foundation for Medical Research. If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures




References
-
- Anders H.J., Saxena R., Zhao M.H., Parodis I., Salmon J.E., Mohan C. Lupus nephritis. Nat. Rev. Dis. Primers. 2020;6:7. - PubMed
-
- Siegel C.H., Sammaritano L.R. Systemic lupus erythematosus: a review. JAMA. 2024;331:1480–1491. - PubMed
-
- Flores-Mendoza G., Sanson S.P., Rodriguez-Castro S., Crispin J.C., Rosetti F. Mechanisms of tissue injury in lupus nephritis. Trends Mol. Med. 2018;24:364–378. - PubMed
-
- Narasimhan P.B., Marcovecchio P., Hamers A.A.J., Hedrick C.C. Nonclassical monocytes in health and disease. Annu. Rev. Immunol. 2019;37:439–456. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials

