Navigating the brain: Harnessing endogenous cellular hitchhiking for targeting neoplastic and neuroinflammatory diseases
- PMID: 40453753
- PMCID: PMC12125590
- DOI: 10.1016/j.ajps.2025.101040
Navigating the brain: Harnessing endogenous cellular hitchhiking for targeting neoplastic and neuroinflammatory diseases
Abstract
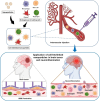
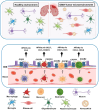
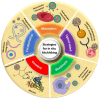
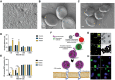
Cellular hitchhiking is an emerging therapeutic strategy that uses an endogenous cell migration mechanism to deliver therapeutics to specific sites in the body. Owing to the low permeability and presence of the blood-brain barrier (BBB), the targeted delivery of therapeutics is limited, leading to inadequate localization in the brain. NCs fail to extravasate significantly into the tumor microenvironment (TME), demonstrating poor accumulation and tumor penetration. The novel cellular hitchhiking concept has been utilized to promote systemic half-life and therapeutic targeting. Neoplastic and neuroinflammatory diseases of the brain, including glioblastoma and neuroinflammation, face critical hurdles for efficiently delivering therapeutic entities owing to the BBB. Cellular hitchhiking can surmount these hurdles by utilizing various cell populations, such as stem cells, monocytes/macrophages, neutrophils, and platelets, as potential functional carriers to deliver the therapeutic cargo through the BBB. These carrier cells have the innate capability to traverse the BBB, transit through the brain parenchyma, and specifically reach disease sites such as inflammatory and neoplastic lesions owing to chemotactic navigation, i.e., movement attributed to chemical stimuli. Chemotherapeutic drugs delivered by cellular hitchhiking to achieve tumor-specific targeting have been discussed. This article explores various cell types for hitchhiking NCs to the TME with in-depth mechanisms and characterization techniques to decipher the backpack dissociation dynamics (nanoparticle payload detachment characteristics from hitchhiked cells) and challenges toward prospective clinical translation.
Keywords: Brain-targeting; Cellular hitchhiking; Glioblastoma.
© 2025 Shenyang Pharmaceutical University. Published by Elsevier B.V.
Conflict of interest statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
Figures



References
-
- Liu Q., Li X., Wang Y., Liu X., Cao Y., Zhang H., et al. Neutrophil hitchhiking for nanoparticle delivery to the central nervous system. Appl Mater Today. 2024;38
-
- Kaur N., Chugh H., Sakharkar M.K., Dhawan U., Chidambaram S.B., Chandra R. Neuroinflammation mechanisms and phytotherapeutic intervention: a systematic review. ACS Chem Neurosci. 2020;11:3707–3731. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

