Coaggregation of oral pathogens by postbiotic lactobacilli
- PMID: 40453788
- PMCID: PMC12123943
- DOI: 10.1080/20002297.2025.2508483
Coaggregation of oral pathogens by postbiotic lactobacilli
Abstract
Introduction: Coaggregation may reduce the abundance of bacteria in physiological fluids, such as saliva, as aggregated bacteria are cleared more easily than planktonic cells. This study aimed to identify Lactobacillus strains that coaggregate with oral pathogens with the perspective of using this approach to improve oral health.
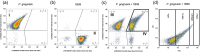
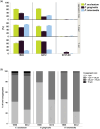
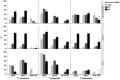
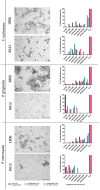
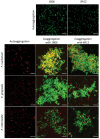
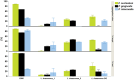
Material and methods: Coaggregation of 719 postbiotic Lactobacillus strains with target pathogens Fusobacterium nucleatum, Porphyromonas gingivalis, and Prevotella intermedia was quantified by absorbance. Coaggregation efficacy of selected strains with clinical isolates and in the presence of other salivary bacteria was determined by flow cytometry. Brightfield and confocal microscopy were applied to characterize the size and structure of coaggregates. Pangenome analysis was used to identify genomic regions potentially involved in the coaggregation activity.
Results: Two strains, Lacticaseibacillus rhamnosus 1B06 and Lacticaseibacillus paracasei 8A12, coaggregated efficiently with all three target pathogens and clinical isolates of the same species even in the presence of other salivary bacteria. The coaggregation capability of the selected Lactobacillus strains was unique and could not be reproduced with other genetically similar lactic acid bacteria of the same species.
Conclusion: Lactobacillus strains capable of coaggregating oral pathogens were identified as promising candidates for the development of new postbiotic ingredients for oral hygiene products.
Keywords: Fusobacterium nucleatum; Porhyromonas gingivalis; Postbiotic; gingivitis; halitosis; lactobacillus; malodor; oral health; screening.
© 2025 The Author(s). Published by Informa UK Limited, trading as Taylor & Francis Group.
Conflict of interest statement
The authors declare that the study was funded by Novozymes A/S and that K. Tykwinska, P. Golletz, M. Collignon and C. Hall are employees of Novonesis A/S.
Figures





References
LinkOut - more resources
Full Text Sources
