HMGB1 Inhibition Alleviates Chronic Nonbacterial Prostatitis by Suppressing M1 Polarization of Macrophages
- PMID: 40453963
- PMCID: PMC12123095
- DOI: 10.2147/JIR.S502616
HMGB1 Inhibition Alleviates Chronic Nonbacterial Prostatitis by Suppressing M1 Polarization of Macrophages
Abstract
Background and objective: Chronic Prostatitis/Chronic Pelvic Pain Syndrome (CP/CPPS) poses a significant threat to male urinary health and has an unclear pathogenesis. High-mobility group box 1 (HMGB1), a danger-associated molecular pattern that has been identified as a key mediator in various inflammatory diseases. However, its role in CP/CPPS remains unclear. This study aimed to investigate HMGB1's potential contributions to the pathogenesis of CP/CPPS, offering new perspectives for innovative treatments.
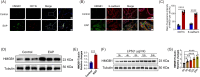
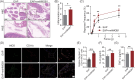
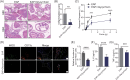
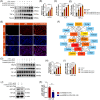
Materials and methods: We have successfully extracted prostate antigens from Sprague-Dawley rat prostate tissue and established an experimental autoimmune prostatitis (EAP) mouse model in non-obese diabetic (NOD) mice. Subsequently, EAP mice were treated with recombinant HMGB1 protein (rmHMGB1) or the HMGB1-specific inhibitor glycyrrhizin for 14 days. Behavioral test was performed to assess the chronic pelvic pain. Hematoxylin and eosin (H&E) staining was employed to assess the extent of inflammatory cell infiltration in the prostate, and enzyme-linked immunosorbent assay (ELISA) was performed to assess levels of inflammatory cytokines. Co-immunofluorescence was used to analyze the functional phenotype of macrophages and spatial localization of HMGB1 in prostate of EAP mice. To further validate these findings, we conducted in vitro experiments. In these experiments, lipopolysaccharide (LPS) was used to induce an inflammatory environment in RAW264.7 cells. Interventions included administering rmHMGB1, silencing HMGB1 gene expression with siRNA, and treating cells with the TRAF6 inhibitor C25-140. After interventions, Western blot and immunofluorescence were employed to evaluate the impact on M1 macrophage polarization and inflammation.
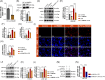
Results: In this study, we demonstrate that HMGB1 is highly expressed in the prostate tissues of EAP mice. Treating EAP mice with rmHMGB1 significantly increased prostate histological scores (2.83 vs 1.83, p < 0.05) and the sensitivity to pain stimuli, as evidenced by a higher response frequency to von Frey filament stimulation at 4 g (68.33% vs 53.33%, p < 0.05). This treatment also increased the levels of inflammatory proteins IL-6 and TNF-α. In contrast, suppressing HMGB1 with glycyrrhizin significantly reduced inflammation, as indicated by decreased histological scores (0.50 vs 2.17, p < 0.05), and attenuated pain sensitivity, as evidenced by a lower response frequency to von Frey filament stimulation at 4 g (30.83% vs 52.50%, p < 0.05). Glycyrrhizin treatment also reduced IL-6 and TNF-α levels. Furthermore, the proportion of CD11b+iNOS+ cells, indicative of M1 macrophage polarization, was significantly reduced after glycyrrhizin treatment. In vitro, HMGB1 can regulate the activity of TRAF6 by partially modulating its ubiquitination and degradation, thereby amplifying TRAF6-mediated NF-κB activation, promoting M1 macrophage polarization, and exacerbating inflammation.
Discussion and conclusions: HMGB1 can enhance TRAF6-mediated NF-κB activation, thereby driving M1 macrophage polarization and exacerbating prostate inflammation in EAP mice. Inhibiting HMGB1 expression with glycyrrhizin can suppress M1 polarization of macrophages to alleviate prostate inflammation. This study suggests that targeting the HMGB1/TRAF6/NF-κB signaling pathway may be an effective therapeutic approach for CP/CPPS.
Keywords: HMGB1; TRAF6; chronic nonbacterial prostatitis; inflammation; macrophages.
© 2025 Zhou et al.
Conflict of interest statement
The authors declare no conflicts of interest in this work.
Figures


References
-
- Baiyi L, Hongbin C, Wen H, et al. Protective effect of bamboo shoot oil on experimental nonbacterial prostatitis in rats. Food Chem. 2011;124(3):1017–1023. doi: 10.1016/j.foodchem.2010.07.066 - DOI
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

