Lipidomics-based plasma signature of alcohol-related hepatitis linked to short-term mortality
- PMID: 40453976
- PMCID: PMC12123345
- DOI: 10.1016/j.jhepr.2025.101367
Lipidomics-based plasma signature of alcohol-related hepatitis linked to short-term mortality
Abstract
Background & aims: Severe alcohol-related hepatitis (sAH) is an inflammatory condition with high short-term mortality. Hypothesis-driven approaches have failed to identify effective treatments. Given the role of lipids as inflammatory mediators, this study aimed to identify lipidomic changes and lipid species associated with sAH and mortality risk.
Methods: Untargeted lipidomics was performed on serum samples from two cohorts of patients with sAH and decompensated cirrhosis (DC). Principal component analysis and orthogonal partial least squares discriminant analysis were used to assess lipidome changes. Correlations were made with lipoproteins, lipid mediators, cytokines, cytokeratin fragments, and histological indices.
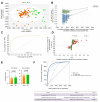
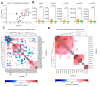
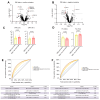
Results: In the first part, 78 patients with sAH were matched on bilirubin levels with 23 patients with DC. Lipidomics identified a distinct sAH signature involving glycerophospholipids, including PC(34:2) (odds ratio [OR] 2.18, 95% confidence interval [CI] 1.45-7.05, p = 0.01), PC(O-38:5) (OR 3.31, 95% CI 2.23-7.14, p = 0.002), PI(38:4) (OR 0.71, 95% CI 0.46-0.88, p = 0.02), and LPC(18:1) (OR 0.47, 95% CI 0.32-0.82, p = 0.01). These lipids demonstrated excellent discriminatory power between sAH and DC with areas under the receiver operating characteristic curve (AUROCs) between 0.87 and 0.88. In the second part, in 159 sAH patients, specific lipids, including carnitines CAR(2:0) (OR 2.51, 95% CI 1.25-4.96, p = 0.008) and CAR(16:1) (OR 2.21, 95% CI 1.09-7.48, p = 0.009), were linked to 90-day mortality. Acylcarnitines correlated with disease severity parameters such as model for end-stage liver disease, pro-inflammatory cytokines levels, and hepatocyte ballooning on pathology.
Conclusions: Untargeted lipidomics identified a glycerophospholipid and sphingolipid signature distinguishing sAH from DC, implicating lipid species involved in liver regeneration and immune function. Acylcarnitine accumulation in patients with sAH and poor prognosis suggests mitochondrial dysfunction and warrants further investigation into therapeutic potential.
Impact and implications: Lipids can act as mediators at the interface between the immune system and metabolism, potentially contributing to the pathogenesis and outcomes of patients with severe alcohol-related hepatitis, prompting us to investigate lipidomic changes in this population using untargeted approaches, compared with patients with decompensated cirrhosis. This study highlights a distinct lipidomic signature in patients with severe alcohol-related hepatitis compared with decompensated cirrhosis, primarily involving glycerophospholipids and sphingolipids. Specific lipid classes, such as acylcarnitines, suggest significant mitochondrial dysfunction and are associated with disease severity and short-term mortality in patients with severe alcohol-related hepatitis. These findings underscore the importance of targeted investigations into these lipid species, their pathways, and their links to disease severity and outcomes, particularly in this condition that currently lacks specific treatments.
Keywords: Acute-on-chronic liver failure; Acylcarnitines; Alcohol-related hepatitis; Cirrhosis; Glycerophospholipids; Lipidomics; Lysophospholipids; Mitochondrial dysfunction.
© 2025 The Authors.
Conflict of interest statement
The authors declare no conflicts of interest that pertain to this work. Please refer to the accompanying ICMJE disclosure forms for further details.
Figures


References
-
- Mathurin P., Thursz M. Endpoints and patient stratification in clinical trials for alcoholic hepatitis. J Hepatol. 2019;70:314–318. - PubMed
-
- Szabo G., Thursz M., Shah V.H. Therapeutic advances in alcohol-associated hepatitis. J Hepatol. 2022;76:1279–1290. - PubMed
-
- Rattan P., Shah V.H. Review article: current and emerging therapies for acute alcohol-associated hepatitis. Aliment Pharmacol Ther. 2022;56:28–40. - PubMed
-
- Louvet A., Thursz M.R., Kim D.J., et al. Corticosteroids reduce risk of death within 28 days for patients with severe alcoholic hepatitis, compared with pentoxifylline or placebo—a meta-analysis of individual data from controlled trials. Gastroenterology. 2018;155:458–468.e8. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

