Production of a Recombinant Fibrinolytic Protease from an Isolate of Serratia marcescens from the Amazon Basin
- PMID: 40454077
- PMCID: PMC12123961
- DOI: 10.1021/acsomega.5c02194
Production of a Recombinant Fibrinolytic Protease from an Isolate of Serratia marcescens from the Amazon Basin
Abstract
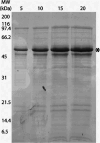
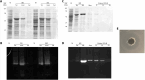
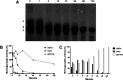
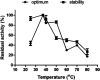
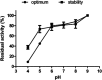
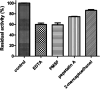
Intravenous fibrinolytic agents are essential for the treatment of cardiovascular diseases, acting through plasminogen activation to dissolve thrombi. However, current therapies are often limited by low fibrin specificity, high production costs, and side effects such as bleeding and allergic reactions. In this study, we describe a novel fibrinolytic protease, rSM519, derived from Serratia marcescens CBAM 519 and recombinantly expressed in Escherichia coli. The enzyme was purified via affinity chromatography and exhibited a molecular mass of 56 kDa. Biochemical assays revealed that rSM519 is a metalloprotease with optimal activity at pH 9.0 and 37 °C, significantly enhanced by Mn2+ ions. Unlike conventional agents such as tissue plasminogen activator or streptokinase, rSM519 acts directly on fibrin without requiring plasminogen activation. It efficiently degraded the α and β chains of fibrinogen, mimicking plasmin-like behavior, and showed no hemolytic activity. These features position rSM519 as a promising thrombolytic candidate with potential advantages over existing therapies, including lower production costs, reduced side effects, and direct fibrin-targeting activity.
© 2025 The Authors. Published by American Chemical Society.
Figures


References
-
- Kim H. C., Choi B.-S., Sapkota K., Kim S., Lee H. J., Yoo J. C., Kim S.-J.. Purification and Characterization of a Novel, Highly Potent Fibrinolytic Enzyme from Paecilomyces Tenuipes. Process Biochem. 2011;46(8):1545–1553. doi: 10.1016/j.procbio.2011.04.005. - DOI
LinkOut - more resources
Full Text Sources
