High-Resolution HPLC for Separating Peptide-Oligonucleotide Conjugates
- PMID: 40454080
- PMCID: PMC12120573
- DOI: 10.1021/acsomega.5c01308
High-Resolution HPLC for Separating Peptide-Oligonucleotide Conjugates
Abstract
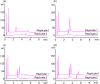
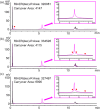
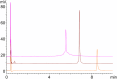
Peptide-oligonucleotide conjugates (POCs) are chimeric molecules that combine the specificity of oligonucleotides with the functionality of peptides, improving the delivery and therapeutic potential of nucleic acid-based drugs. However, the analysis of POCs, particularly those containing arginine-rich sequences, poses major challenges because of aggregation caused by electrostatic interactions. In this study, we developed an optimized high-performance liquid chromatography (HPLC) method for analyzing POCs. Using a conjugate of DNA and nona-arginine as a model compound, we systematically investigated the effects of various analytical parameters, including column type, column temperature, mobile-phase composition, and pH. A column packed with C18 resin with wide pores combined with butylammonium acetate as the ion-pairing reagent and an optimal column temperature of 80 °C provided superior peak resolution and sensitivity. The optimized conditions gave clear separation of POCs from unlinked oligonucleotides and enabled the detection of nucleic acid fragments lacking an alkyne moiety as a linkage part, which is critical for quality control. Our HPLC method is robust and reproducible and substantially reduces the complexity, time, and cost associated with the POC analysis. The method may improve the efficiency of quality control in the production of POCs, thereby supporting their development as promising therapeutic agents for clinical applications.
© 2025 The Authors. Published by American Chemical Society.
Figures




Similar articles
-
Versatile phosphoramidation reactions for nucleic acid conjugations with peptides, proteins, chromophores, and biotin derivatives.Bioconjug Chem. 2010 Sep 15;21(9):1642-55. doi: 10.1021/bc1001505. Bioconjug Chem. 2010. PMID: 20690641
-
High perfomance liquid chromatography in pharmaceutical analyses.Bosn J Basic Med Sci. 2004 May;4(2):5-9. doi: 10.17305/bjbms.2004.3405. Bosn J Basic Med Sci. 2004. PMID: 15629016 Free PMC article.
-
Evaluation of ion pairing reversed-phase liquid chromatography for the separation of large RNA molecules.J Chromatogr A. 2025 Jan 11;1740:465574. doi: 10.1016/j.chroma.2024.465574. Epub 2024 Dec 2. J Chromatogr A. 2025. PMID: 39644744
-
Peptide-Oligonucleotide Conjugation: Chemistry and Therapeutic Applications.Curr Issues Mol Biol. 2024 Sep 30;46(10):11031-11047. doi: 10.3390/cimb46100655. Curr Issues Mol Biol. 2024. PMID: 39451535 Free PMC article. Review.
-
Emerging applications of peptide-oligonucleotide conjugates: bioactive scaffolds, self-assembling systems, and hybrid nanomaterials.Org Biomol Chem. 2019 Feb 13;17(7):1668-1682. doi: 10.1039/c8ob02436g. Org Biomol Chem. 2019. PMID: 30483688 Review.
References
LinkOut - more resources
Full Text Sources
