A Retrospective Test-Negative Case-Control Study to Evaluate Influenza Vaccine Effectiveness in Preventing Influenza Among Immunocompromised Adults With a Solid Organ Transplant
- PMID: 40454297
- PMCID: PMC12124284
- DOI: 10.3389/ti.2025.14187
A Retrospective Test-Negative Case-Control Study to Evaluate Influenza Vaccine Effectiveness in Preventing Influenza Among Immunocompromised Adults With a Solid Organ Transplant
Abstract
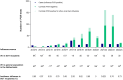
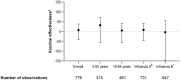
Vaccination may prevent influenza in solid organ transplant (SOT) recipients. This study evaluates the influenza vaccine effectiveness (VE) in this high-risk population in the Netherlands. We also compared disease progression and 30-day mortality between vaccinated and unvaccinated influenza patients. In this multicenter, test-negative case-control study, SOT recipients with respiratory symptoms were included when tested for viral respiratory infections during the respiratory seasons between 1 January 2013 and 1 July 2024. Cases had a positive influenza PCR, while controls tested negative. Influenza vaccination in cases (74/174) and controls (291/602) were compared after adjusting for potential confounders. VE was calculated as (1-adjusted odds ratio) x 100. The overall VE was 6.9% (95% CI -40.9 to 38.4), with considerable variation across seasons. For those aged ≥65 years, VE was higher (32.4%, 95% CI -56.5-70.8) compared to those aged 18-64 years (4.8%, 95% CI -56.5 to 42.1). The adjusted VE against influenza A [7.5% (-46.0 to 41.3)] was higher than against influenza B (-3.8% (-146.7 to 56.3)). No differences in influenza-related complications were observed between the vaccinated and unvaccinated cases. The observed seasonal influenza vaccine effectiveness in adult SOT recipients is limited; further investigation for improvement is warranted.
Keywords: Netherlands; influenza; influenza vaccination; influenza vaccine effectiveness; solid organ transplant patients.
Copyright © 2025 Prins, van Dokkum, de Vries, Tushuizen, van der Helm, Spithoven, van der Meer, Groeneveld, Visser, le Cessie, Vollaard and Groeneveld.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- WHO. Influenza (Seasonal) (2023). Available online at: https://www.who.int/news-room/fact-sheets/detail/influenza-(seasonal) (Accessed November 21, 2023).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

