Comparative analysis of endophytic bacterial localization and microbiome diversity in plant varieties under varied growth conditions through microscopic imaging and sequencing techniques
- PMID: 40454365
- PMCID: PMC12122750
- DOI: 10.3389/fmicb.2025.1568209
Comparative analysis of endophytic bacterial localization and microbiome diversity in plant varieties under varied growth conditions through microscopic imaging and sequencing techniques
Abstract
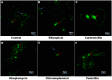
Introduction: Intracellular colonization by endophytic bacteria (EB) is a relatively new and less explored aspect of plant microbiome research. In this study, we investigated the presence and localization of EB in Nicotiana tabacum var. Podali and Vigna radiata var. Pratap using SYTO9 (S9) and Propidium Iodide (PI) staining.
Methodology: Confocal Laser Scanning Microscopy (CLSM) was used to visualized bacterial localization, MitoTracker Deep Red (MDR) was used to confirm non- overlapping mitochondrial staining. Time-lapse imaging was employed to observe bacterial motility. For microbial community profiling next-generation sequencing (NGS) of the 16S rRNA gene was conducted to analyze bacterial diversity and composition.
Results: Confocal laser scanning microscopy (CLSM) revealed S9-labelled live bacteria located close to the nucleus in Podali tissues and suspension cultures, while PI selectively stained dead cells. MitoTracker Deep Red (MDR) confirmed that there was no overlap with mitochondrial staining. Interestingly, time-lapse imaging captured the movement of bacteria within the cells, indicating possible bacterial motility. EB were observed in both in vitro and field-grown Podali plants, whereas they were detected only in field-grown Pratap plants. Next-generation sequencing revealed that Podali harbored a much higher bacterial diversity, with 37 bacterial families identified mainly from Burkholderiaceae and Enterobacteriaceae. In contrast, Pratap plants showed lower diversity, with only 10 bacterial families, dominated by Rhizobiaceae.
Conclusion: This study is among the first to report intracellular EB localization in these plant varieties and demonstrates how environmental conditions and growth methods can influence the composition of plant-associated microbiomes.
Keywords: endophytic bacteria; intracellular colonization; microbiome diversity; next-generation sequencing; plant-microbe interactions.
Copyright © 2025 Shadab, Samanta, Baruah, Deka, Raj and Talukdar.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





References
-
- Akinde S. B., Sunday A. A., Adeyemi F. M., Fakayode I. B., Oluwajide O. O., Adebunmi A. A., et al. . (2016). Microbes in irrigation water and fresh vegetables: potential pathogenic bacteria assessment and implications for food safety. Appl. Biosaf. 21, 89–97. doi: 10.1177/1535676016652231 - DOI
-
- Bader C., Sorvina A., Darby J., Lock M., Soo J. Y., Johnson I., et al. . (2018). Imaging mitochondria live or fixed muscle tissues. Protocol Exchange, 1–13. doi: 10.1038/protex.2018.101, PMID: - DOI
-
- Bianciotto V., Genre A., Jargeat P., Lumini E., Becard G., Bonfante P. (2004). Vertical transmission of Endobacteria in the Arbuscular Mycorrhizal fungus Gigaspora margarita through generation of vegetative spores. Appl. Environ. Microbiol. 70, 3600–3608. doi: 10.1128/AEM.70.6.3600-3608.2004, PMID: - DOI - PMC - PubMed
-
- Chávez-González J. D., Flores-Núñez V. M., Merino-Espinoza I. U., Partida-Martínez L. P. (2024). Desert plants, arbuscular mycorrhizal fungi and associated bacteria: exploring the diversity and role of symbiosis under drought. Environ. Microbiol. Rep. 16, 1–17. doi: 10.1111/1758-2229.13300, PMID: - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

