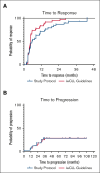
Frequent response monitoring in chronic lymphocytic leukemia clinical trials: what is the value?
- PMID: 40454398
- PMCID: PMC12082098
- DOI: 10.1016/j.bneo.2024.100006
Frequent response monitoring in chronic lymphocytic leukemia clinical trials: what is the value?
Conflict of interest statement
Conflict-of-interest disclosure: A.Ö. and M.P. have received unrestricted scientific grants from 10.13039/100017239BeiGene. The remaining authors declare no competing financial interests.
Figures
References
-
- Hallek M, Cherson BD, Catovsky D, et al. IwCLL guidelines for diagnosis, indications for treatment, response assessment, and supportive management of CLL. Blood. 2018;131(25):2745–2760. - PubMed
-
- Eichhorst BF, Fischer K, Fink AM, et al. Limited clinical relevance of imaging techniques in the follow-up of patients with advanced chronic lymphocytic leukemia: results of a meta-analysis. Blood. 2011;117(6):1817–1821. - PubMed
-
- Ghia P, Pluta A, Wach M, et al. ASCEND: phase III, randomized trial of acalabrutinib versus idelalisib plus rituximab or bendamustine plus rituximab in relapsed or refractory chronic lymphocytic leukemia. J Clin Oncol. 2020;38(25):2849–2861. - PubMed
-
- O’Brien S, Jones JA, Coutre SE, et al. Ibrutinib for patients with relapsed or refractory chronic lymphocytic leukaemia with 17p deletion (RESONATE-17): a phase 2, open-label, multicentre study. Lancet Oncol. 2016;17(10):1409–1418. - PubMed
-
- Tam CS, Brown JR, Kahl BS, et al. Zanubrutinib versus bendamustine and rituximab in untreated chronic lymphocytic leukaemia and small lymphocytic lymphoma (SEQUOIA): a randomized, controlled, phase 3 trial. Lancet Oncol. 2022;23(8):1031–1043. - PubMed
LinkOut - more resources
Full Text Sources