4E-BP1-dependent translation in microglia controls mechanical hypersensitivity in male and female mice
- PMID: 40454470
- PMCID: PMC12126233
- DOI: 10.1172/JCI180190
4E-BP1-dependent translation in microglia controls mechanical hypersensitivity in male and female mice
Abstract
Spinal microglia play a pivotal role in the development of neuropathic pain. Peripheral nerve injury induces changes in the transcriptional profile of microglia, including increased expression of components of the translational machinery. Whether microglial protein synthesis is stimulated following nerve injury and has a functional role in mediating pain hypersensitivity is unknown. Here, we show that nascent protein synthesis is upregulated in spinal microglia following peripheral nerve injury in both male and female mice. Stimulating mRNA translation in microglia by selectively ablating the translational repressor eukaryotic initiation factor 4E-binding protein 1 (4E-BP1) promoted the transition of microglia to a reactive state and induced mechanical hypersensitivity in both sexes, whereas spontaneous pain was increased only in males. Conversely, inhibiting microglial translation by expressing a mutant form of 4E-BP1 in microglia attenuated their activation following peripheral nerve injury and alleviated neuropathic pain in both sexes. Thus, stimulating 4E-BP1-dependent translation promotes microglial reactivity and mechanical hypersensitivity, whereas inhibiting it alleviates neuropathic pain.
Keywords: Cell biology; Neuroscience; Pain.
Conflict of interest statement
Figures
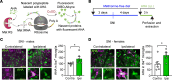



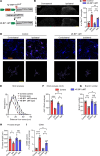
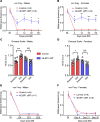
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

