Mechanism-based nonopioid analgesic targets
- PMID: 40454476
- PMCID: PMC12126239
- DOI: 10.1172/JCI191346
Mechanism-based nonopioid analgesic targets
Abstract
Acute pain management has historically been dominated by opioids, whose efficacy is overshadowed by the risks of addiction, tolerance, and dependence, culminating in the global opioid crisis. To transcend this issue, we must innovate beyond opioid-based μ receptor treatments, identifying nonopioid analgesics with high efficacy and minimal adverse effects. This Review navigates the multifaceted landscape of inflammatory, neuropathic, and nociplastic pain, emphasizing mechanism-based analgesic targets tailored to specific pain conditions. We delve into the challenges and breakthroughs in clinical trials targeting ion channels, GPCRs, and other molecular targets. We also highlight the intricate crosstalk between different physiological systems and the need for multimodal interventions with distinct pharmacodynamics to manage acute and chronic pain, respectively. Furthermore, we explore emerging strategies, including gene therapy, stem cell therapy, cell type-specific neuromodulation, and AI-driven techniques for objective, unbiased pain assessment and research. These innovative approaches are poised to revolutionize pain management, paving the way for the discovery of safer and more effective analgesics.
Figures
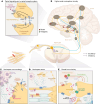
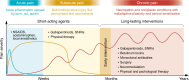
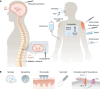
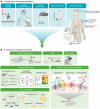
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

