PIEZO1 mediates periostin+ myofibroblast activation and pulmonary fibrosis in mice
- PMID: 40454481
- PMCID: PMC12126248
- DOI: 10.1172/JCI184158
PIEZO1 mediates periostin+ myofibroblast activation and pulmonary fibrosis in mice
Abstract
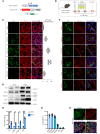
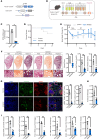
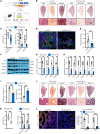
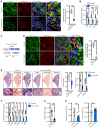
Idiopathic pulmonary fibrosis (IPF) is a devastating interstitial lung disease characterized by the excessive accumulation of activated myofibroblasts that deposit extracellular matrix (ECM) protein, leading to progressive scar formation and mechanical stress. However, the cellular origin and fate of myofibroblasts remain controversial, and the mechanisms by which myofibroblasts sense mechanical cues in the lung are unclear. Here, we report that periostin (Postn) is a reliable and distinctive marker for pulmonary myofibroblasts, while ablation of Postn+ myofibroblasts after injury ameliorated lung fibrosis. PIEZO1 was highly expressed in Postn+ myofibroblast and played a vital role in mechanoactivation of Postn+ myofibroblast and development of lung fibrosis. Conditional deletion of Piezo1 in Postn+ myofibroblasts significantly inhibited lung fibrosis by suppressing myofibroblast activation and proliferation. Loss of Piezo1 led to disruption of actin organization and prevention of Yap/Taz nuclear localization, thus shifting the myofibroblasts from a proliferative state into a stressed and apoptotic state. Furthermore, myofibroblast-specific Yap/Taz deletion fully recapitulated the protective phenotypes of myofibroblast-Piezo1-KO mice. These findings show that periostin marks pulmonary myofibroblasts, and that PIEZO1-mediated mechanosensation is essential for myofibroblast activation in the lung. Targeting PIEZO1 in the periostin-expressing cells is a novel therapeutic option to interfere with fibrotic diseases such as IPF .
Keywords: Cell biology; Fibrosis; Ion channels; Pulmonology.
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

