Hepatic glycogen directly regulates gluconeogenesis through an AMPK/CRTC2 axis in mice
- PMID: 40454488
- PMCID: PMC12126231
- DOI: 10.1172/JCI188363
Hepatic glycogen directly regulates gluconeogenesis through an AMPK/CRTC2 axis in mice
Abstract
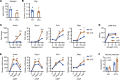
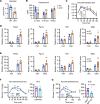
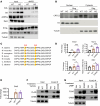
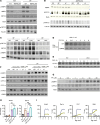
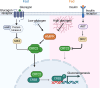
Glycogenolysis and gluconeogenesis ensure sufficient hepatic glucose production during energy shortages. Here, we report that hepatic glycogen levels control the phosphorylation of a transcriptional coactivator to determine the amplitude of gluconeogenesis. Decreased liver glycogen during fasting promotes gluconeogenic gene expression, while feeding-induced glycogen accumulation suppresses it. Liver-specific deletion of the glycogen scaffolding protein, protein targeting to glycogen (PTG), reduces glycogen levels, increases the expression of gluconeogenic genes, and promotes glucose production in primary hepatocytes. In contrast, liver glycogen phosphorylase (PYGL) knockdown or inhibition increases glycogen levels and represses gluconeogenic gene expression. These changes in hepatic glycogen levels are sensed by AMP-activated protein kinase (AMPK). AMPK activity is increased when glycogen levels decline, resulting in the phosphorylation and stabilization of CREB-regulated transcriptional coactivator 2 (CRTC2), which is crucial for the full activation of the cAMP-responsive transcriptional factor CREB. High glycogen allosterically inhibits AMPK, leading to CRTC2 degradation and reduced CREB transcriptional activity. Hepatocytes with low glycogen levels or high AMPK activity show higher CRTC2 protein levels, priming the cell for gluconeogenesis through transcriptional regulation. Thus, glycogen plays a regulatory role in controlling hepatic glucose metabolism through the glycogen/AMPK/CRTC2 signaling axis, safeguarding efficient glucose output during fasting and suppressing it during feeding.
Keywords: Cell biology; Gluconeogenesis; Glucose metabolism; Metabolism; Signal transduction.
Conflict of interest statement
Figures


References
-
- Berman HK, et al. Overexpression of protein targeting to glycogen (PTG) in rat hepatocytes causes profound activation of glycogen synthesis independent of normal hormone- and substrate-mediated regulatory mechanisms. J Biol Chem. 1998;273(41):26421–26425. doi: 10.1074/jbc.273.41.26421. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

