Nanoparticle Therapeutics in Clinical Perspective: Classification, Marketed Products, and Regulatory Landscape
- PMID: 40454890
- PMCID: PMC12288819
- DOI: 10.1002/smll.202502315
Nanoparticle Therapeutics in Clinical Perspective: Classification, Marketed Products, and Regulatory Landscape
Abstract
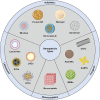
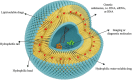
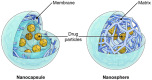
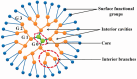
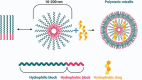
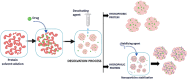
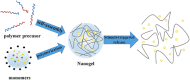
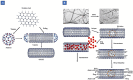
Nanoparticle-based therapeutics, emerging from advances in nanotechnology, outperform traditional drug therapies by virtue of their distinct biological properties that enhance therapeutic efficacy, reduce toxicity, and enable precise targeting. Since the 1980s, the number of nanoparticle-based pharmaceutical products has expanded considerably, capturing a significant portion of the pharmaceutical market. These systems function as therapeutic agents or as vehicles for delivering active pharmaceutical or diagnostic compounds to targeted areas. However, despite their transformative potential, the development of comprehensive and harmonized regulatory frameworks for nanomedicines remains a critical challenge. This review provides a current overview of market-approved nanoparticle therapeutics, analyzing global regulatory strategies, including pre-clinical testing, safety assessments, manufacturing processes, and quality control standards. By discussing the existing shortcomings, this review highlights the importance of adaptive regulatory pathways in a global context. It aims to support researchers and stakeholders in navigating the regulatory landscape, facilitating the successful commercialization and clinical translation of nanoparticle-based therapeutics.
Keywords: marketed products; nanomedicines; nanoparticles; regulatory approval; regulatory guidelines.
© 2025 The Author(s). Small published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

