Adjunctive middle meningeal artery embolization for non-acute subdural hematoma: A GRADE-assessed meta-analysis and trial sequential analysis on randomized trials
- PMID: 40455367
- PMCID: PMC12129861
- DOI: 10.1007/s00701-025-06574-9
Adjunctive middle meningeal artery embolization for non-acute subdural hematoma: A GRADE-assessed meta-analysis and trial sequential analysis on randomized trials
Abstract
Background and purpose: Non-acute subdural hematoma (NASDH) is a prevalent neurological condition, encompassing chronic and subacute types. Despite standard-care, including surgical evacuation and medical management, recurrence rates remain high. Emerging evidence suggests that middle meningeal artery embolization (MMAE) as an adjunctive procedure may reduce recurrence. This study evaluates the efficacy and safety of MMAE in NASDH.
Methods: A systematic review and meta-analysis of randomized controlled trials (RCTs) retrieved from PubMed, EMBASE, WOS, Scopus, and Cochrane until November 2024. The analysis presented risk ratios (RR) for dichotomous outcomes and mean differences (MD) for continuous outcomes, with 95% confidence intervals (CI) using R software. The GRADE system assessed evidence certainty, alongside trial sequential analysis for result reliability.
Prospero id: CRD42024625504.
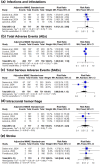
Results: Six RCTs and 1,544 patients were included, with an average of 4.7 months follow-up. Adjunctive MMAE, compared to standard-care, significantly reduced hematoma recurrence (8% vs 15.6%; RR: 0.52; 95% CI: [0.37:0.73]; P < 0.01) and surgical rescue (4.5% vs. 12.7%; RR: 0.36; 95% CI: [0.25:0.53]; P < 0.01). However, no significant effect was found for recurrence without surgery (P = 0.94), hematoma volume (P = 0.18), thickness (P = 0.34), or hospital stay (P = 0.37). Infection rates were higher with MMAE (8.4% vs. 4.8%; RR: 1.81; 95% CI: [1.23:2.66]; P < 0.01), but adverse events (AEs), serious AEs, intracranial hemorrhage, stroke, and mortality showed no significant differences.
Conclusion: Adjunctive MMAE reduced hematoma recurrence and surgical rescue rates in NASDH with an acceptable safety profile despite increased infection rates. However, further large-scale trials with extended follow-ups are needed.
Keywords: Brain injury; Clinical trial; Embolization; Neurovascular; Subdural hematoma; Trauma.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests. Ethics Approval: Not applicable. Consent to publish: Not applicable. Consent to participate: Not applicable.
Figures



References
-
- Catapano JS, Koester SW, Srinivasan VM, Rumalla K, Baranoski JF, Rutledge C et al (2022) Total 1-year hospital cost of middle meningeal artery embolization compared to surgery for chronic subdural hematomas: a propensity-adjusted analysis. J Neurointerv Surg 14(8):804–806. 10.1136/neurintsurg-2021-018327 - PubMed
-
- Davies JM, Knopman J, Mokin M, Hassan AE, Harbaugh RE, Khalessi A et al (2024) Adjunctive middle meningeal artery embolization for subdural hematoma. N Engl J Med [Internet] 391(20):1890–1900. 10.1056/NEJMoa2313472 - PubMed
-
- Debs LH, Vale FL, Walker S, Toro D, Mansouri S, Macomson SD et al (2024) Middle meningeal artery embolization following surgical evacuation of symptomatic chronic subdural hematoma improves outcomes, interim results of a prospective randomized trial. J Clin Neurosci 128:110783. 10.1016/j.jocn.2024.110783 - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

