Pharmacokinetics of Atogepant in Healthy Lactating Female Participants: Results from a Phase 1 Lactation Study
- PMID: 40455369
- PMCID: PMC12255638
- DOI: 10.1007/s40120-025-00772-4
Pharmacokinetics of Atogepant in Healthy Lactating Female Participants: Results from a Phase 1 Lactation Study
Abstract
Introduction: Atogepant is approved for the preventive treatment of migraine and is taken orally once daily. This work aimed to characterize the plasma and milk pharmacokinetics of atogepant in lactating females.
Methods: An open-label, phase 1 study (NCT05892757) was conducted in 12 healthy, lactating adult women 1-6 months postpartum from July 11, 2023, to February 22, 2024. A single 60-mg dose of atogepant was administered to participants to determine atogepant's plasma and milk pharmacokinetics, the excretion of atogepant in breast milk, and the relative infant dose (RID). Atogepant was analyzed using validated LC-MS/MS assays in plasma and breast milk samples collected up to 24 h after dosing and during specified intervals through 24 h, respectively. Plasma and milk pharmacokinetic parameters were estimated using non-compartmental methods and compared using linear mixed-effects models. Safety was assessed via adverse event reporting, clinical labs, vital signs, and ECGs throughout the study.
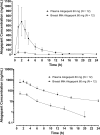
Results: The mean (range) milk-to-plasma ratio for atogepant was 0.076 (0.023-0.104). With nearly undetectable levels of atogepant in breast milk 16-24 h after dosing, the cumulative mean (range) amount of atogepant excreted in breast milk over 24 h was 0.009 mg (0.005-0.016 mg; 0.015% of 60-mg dose), and the mean (range) RID was 0.19% (0.06-0.33%). The mean plasma and milk peak concentrations of atogepant were 779 ng/mL and 57.0 ng/mL, respectively, and the corresponding AUC values were 3270 ng·h/mL and 238 ng·h/mL, respectively. Atogepant exposures in breast milk were 93% lower compared to plasma. Two participants (16.7%) experienced AEs. These included abdominal pain (n = 1) and dyspepsia (n = 1), both of which were non-serious and mild in severity. No new safety signals were identified in this small group of healthy lactating women.
Conclusion: The cumulative mean amount of atogepant recovered in breast milk over 24 h following a 60-mg dose was 0.009 mg, with a RID of 0.19%. CLINICAL TRIAL REGISTRATION: NCT05892757; https://clinicaltrials.gov/study/NCT05892757 .
Keywords: Atogepant; CGRP receptor antagonist; Lactation; Migraine; Milk; Pharmacokinetics; Relative infant dose.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflict of Interest: Ramesh R Boinpally, Jonathan H Smith, and Rosa L De Abreu Ferreira are employees of AbbVie and may hold AbbVie stock or options. Joel M Trugman is a former employee of AbbVie and may hold AbbVie stock or options. Ethical Approval: The studies reported herein were conducted in compliance with the International Council for Harmonisation (ICH) guidelines, guidelines governing clinical study conduct and the ethical principles that have their origin in the Declaration of Helsinki and applicable regulations. Institutional Review Boards and Independent Ethics Committees at participating institutions approved the study prior to study initiation at ICON sites in Salt Lake City, Utah, USA and San Antonio, Texas, USA (Advarra IRB; IRB00000971), and Bio-Kinetic Clinical Applications, LLC in Springfield, Missouri, USA (Bio-Kinetic Clinical Applications IRB; IRB00002771). An informed consent was obtained from all individuals who participated in this study.
Figures

Similar articles
-
Milk and plasma pharmacokinetics of single-dose ubrogepant in healthy lactating women.Headache. 2025 Jul-Aug;65(7):1190-1197. doi: 10.1111/head.14960. Epub 2025 May 20. Headache. 2025. PMID: 40391560 Free PMC article. Clinical Trial.
-
Safety, bactericidal activity, and pharmacokinetics of the antituberculosis drug candidate BTZ-043 in South Africa (PanACEA-BTZ-043-02): an open-label, dose-expansion, randomised, controlled, phase 1b/2a trial.Lancet Microbe. 2025 Feb;6(2):100952. doi: 10.1016/j.lanmic.2024.07.015. Epub 2025 Jan 7. Lancet Microbe. 2025. PMID: 39793592 Clinical Trial.
-
Plasma and Milk Pharmacokinetics and Estimated Milk Withdrawal Time of Tolfenamic Acid in Lactating Sheep.Vet Med Sci. 2024 Nov;10(6):e70047. doi: 10.1002/vms3.70047. Vet Med Sci. 2024. PMID: 39321188 Free PMC article.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
-
Deferasirox for managing iron overload in people with thalassaemia.Cochrane Database Syst Rev. 2017 Aug 15;8(8):CD007476. doi: 10.1002/14651858.CD007476.pub3. Cochrane Database Syst Rev. 2017. PMID: 28809446 Free PMC article.
References
-
- Lipton RB, Halker Singh RB, Mechtler L, et al. Patient-reported migraine-specific quality of life, activity impairment and headache impact with once-daily atogepant for preventive treatment of migraine in a randomized, 52-week trial. Cephalalgia. 2023;51:3331024231190296. 10.1177/03331024231190296. - PubMed
-
- Charles AC, Digre KB, Goadsby PJ, et al. Calcitonin gene-related peptide-targeting therapies are a first-line option for the prevention of migraine: an American Headache Society position statement update. Headache. 2024;64(333–341):20240311. 10.1111/head.14692. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials

