Impaired Cognitive Flexibility With Preserved Learning in an Amyloid Precursor Protein Knock-In Mouse Model of Amyloidopathy
- PMID: 40455650
- PMCID: PMC12129047
- DOI: 10.1111/gbb.70024
Impaired Cognitive Flexibility With Preserved Learning in an Amyloid Precursor Protein Knock-In Mouse Model of Amyloidopathy
Abstract
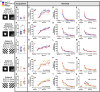
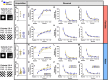
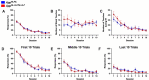
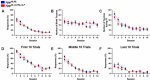
Alzheimer's disease is a debilitating neurodegenerative condition characterized by amyloid beta plaques and tau neurofibrillary tangles, which leads to progressive cognitive decline. Several new mouse models of fast amyloid deposition have been generated with compound mutations, but how these affect high-level cognitive function is still not fully understood. Four cohorts of a second-generation amyloid precursor protein knock-in mouse model, AppNL-G-F/NL-G-F, which develops aggressive amyloidopathy, were compared with two different control groups that do not produce plaques (AppNL/NL and wildtype littermates), on touchscreen-based tests of learning and cognitive flexibility. AppNL-G-F/NL-G-F mice learned to discriminate between two visual stimuli during the pairwise visual discrimination (PVD) task but were impaired when the reward contingencies were reversed (the PVR task). Analyses of the correction trials indicated perseverative behavior. One cohort was further tested on the touchscreen Extinction test, which isolates the ability to withhold responding to a previously rewarded stimulus. The AppNL-G-F/NL-G-F mice extinguished their responding no differently than the AppNL/NL control group. These results indicate that compound mutations in App driving fast accumulation of plaques in this mouse model impair cognitive flexibility and may serve as a preclinical target for putative therapeutic drugs.
Keywords: APP; executive dysfunction; extinction; pairwise visual discrimination; reversal learning.
© 2025 The Author(s). Genes, Brain and Behavior published by International Behavioural and Neural Genetics Society and John Wiley & Sons Ltd.
Conflict of interest statement
T.J.B. and L.M.S. have established a series of targeted cognitive tests for animals, administered via touchscreen within a custom environment known as the “Bussey‐Saksida touchscreen chamber.” Cambridge Enterprise, the technology transfer office of the University of Cambridge, supported commercialization of the Bussey‐Saksida chamber, culminating in a license to Campden Instruments. Any financial compensation received from commercialization of the technology is fully invested in further touchscreen development and/or maintenance. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflicts of interest.
Figures

References
-
- Dementia , “World Health Organization,” 2023, https://www.who.int/news‐room/fact‐sheets/detail/dementia.
-
- McKhann G. M., Knopman D. S., Chertkow H., et al., “The Diagnosis of Dementia due to Alzheimer's Disease: Recommendations From the National Institute on Aging‐Alzheimer's Association Workgroups on Diagnostic Guidelines for Alzheimer's Disease,” Alzheimer's & Dementia 7, no. 3 (2011): 263–269, 10.1016/j.jalz.2011.03.005. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

