Hydroethanolic extract of Schinus terebinthifolia as a promising source of anti-influenza agents: Phytochemical profiling, cheminformatics, molecular docking and dynamics simulations
- PMID: 40455719
- PMCID: PMC12129213
- DOI: 10.1371/journal.pone.0324990
Hydroethanolic extract of Schinus terebinthifolia as a promising source of anti-influenza agents: Phytochemical profiling, cheminformatics, molecular docking and dynamics simulations
Abstract
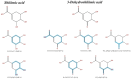
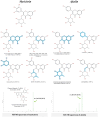
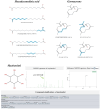
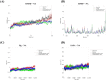
Although Schinus terebinthifolia (commonly known as Brazilian peppertree) has been documented to possess various biological activities, such as anticancer, antibacterial, and antioxidant properties, its anti-influenza activity has not yet been documented. Here, an aqueous-ethanolic extract (30% v/v ethanol solution), prepared from its aerial parts (leaves and stalks), was established to determine whether it is a rich source of antiviral agents. The hydroethanolic plant extract, with a TPC value of 264.11 mg (GAE)/g DE, exhibits a promising IC50 value of 16.33 μg/mL, similar to that of authentic quercetin (IC50 = 12.72 μg/mL), and approximately 5.34 times higher than that of gallic acid (IC50 = 3.06 μg/mL) as determined by the DPPH assay. This extract contains 1.71 mg of gallic acid (representative marker) per gram of dried plant material, according to HPLC analysis. Using untargeted metabolomics analysis coupled with a series of cheminformatics tools (MetFrag, SIRIUS, CSI:FingerID, and CANOPUS), we ultimately proved that the S. terebinthifolia hydroethanolic extract contains simple phenolics (e.g., methyl gallate, ethyl gallate, and chlorogenic acid), flavonoids (afzelin and myricitrin), dicarboxylic acids, and germacrone. As anticipated, the plant extract exhibited anti-influenza activity with an IC50 of 2.21 μg/mL (CC50 > 50 μg/mL) and did not exert hemolytic activity at the concentration of 2000 μg/mL, underscoring its efficacy as a safe antiviral solution. In silico molecular docking and dynamic simulations suggest that neuraminidase and the cap-binding domain of influenza RNA polymerase (PB2) are preferentially targeted for inhibition by the detected metabolites. Owing to the diverse therapeutic effects of secondary metabolites, the anti-H5N1 activity of the newly developed plant extract is currently under investigation.
Copyright: © 2025 Nopkuesuk et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
No authors have competing interests.
Figures











Similar articles
-
Integrative computational analysis of anti-influenza potential in Caesalpinia mimosoides Lamk hydroethanolic extract.Sci Rep. 2025 Feb 1;15(1):3988. doi: 10.1038/s41598-025-87585-5. Sci Rep. 2025. PMID: 39893295 Free PMC article.
-
Unveiling the Potent Antiviral and Antioxidant Activities of an Aqueous Extract from Caesalpinia mimosoides Lamk: Cheminformatics and Molecular Docking Approaches.Foods. 2023 Dec 25;13(1):81. doi: 10.3390/foods13010081. Foods. 2023. PMID: 38201109 Free PMC article.
-
Phytochemical constituents and biological activities of different extracts of Strobilanthes crispus (L.) Bremek leaves grown in different locations of Malaysia.BMC Complement Altern Med. 2015 Nov 27;15(1):422. doi: 10.1186/s12906-015-0873-3. BMC Complement Altern Med. 2015. PMID: 26613959 Free PMC article.
-
In vitro antioxidant potential and in vivo effects of Schinus terebinthifolia Raddi leaf extract in diabetic rats and determination of chemical composition by HPLC-ESI-MS/MS.Nat Prod Res. 2019 Jun;33(11):1655-1658. doi: 10.1080/14786419.2018.1425848. Epub 2018 Jan 18. Nat Prod Res. 2019. PMID: 29347842
-
The genus Schinus (Anacardiaceae): a review on phytochemicals and biological aspects.Nat Prod Res. 2022 Sep;36(18):4839-4857. doi: 10.1080/14786419.2021.2012772. Epub 2021 Dec 9. Nat Prod Res. 2022. PMID: 34886735 Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

