Pre-clinical studies of Schistosoma mansoni vaccines: A scoping review
- PMID: 40455825
- PMCID: PMC12158002
- DOI: 10.1371/journal.pntd.0012956
Pre-clinical studies of Schistosoma mansoni vaccines: A scoping review
Abstract
Background: Schistosomiasis is caused by infection with worms of the genus Schistosoma including S. mansoni. Over 200 million people are infected, sterile immunity does not naturally develop, and no vaccine is available. This could be a critical tool to achieve control and elimination. Numerous candidates have been tested in pre-clinical models, but there is not yet an approved vaccine.
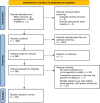
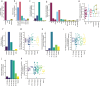
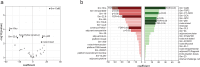
Methodology/principal findings: We conducted a scoping review using a keyword search on Web of Science and a MeSH term search on PubMed. Articles were screened and included if they tested a defined vaccine candidate in a pre-clinical protection assay against S. mansoni between 1994-2024. Vaccine formulation, study design, and efficacy parameters from all articles were extracted. This data was summarised graphically, with the influence of different parameters appraised. A total of 141 candidate antigens were tested in 108 articles over the last 30 years, with most antigens tested only once and three (Sm-CatB, Sm-p80, and Sm-14) tested over 20 times. The median protective efficacy against worms was 35%. 10 antigens achieved over 60% efficacy, and only two (Sm-p80 and Sm-CatB) over 90%. Large variations in efficacy were observed with all repeatedly tested antigens, likely attributable to differing formulations and study designs. The effect of these varying parameters on the resultant efficacy was evaluated.
Conclusions: A few vaccine candidates have achieved promising efficacy in pre-clinical studies. Most vaccines tested however have efficacy that falls short of that required for an impactful schistosomiasis vaccine. The diversity in study designs makes comparing vaccine targets a challenge. Use of consistent and optimized vaccine formulation (including adjuvant and platform) and study design parameters is critical to expedite the development of a schistosome vaccine.
Copyright: © 2025 Houlder et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
-
- Organization WH. Global report on neglected tropical diseases 2024. Geneva: Organization WH. 2024.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

