Dosimetrically coupled multiscale tetrahedral mesh models of human liver vasculature: implications for radiopharmaceutical dosimetry of both organ blood and parenchyma
- PMID: 40456263
- PMCID: PMC12359191
- DOI: 10.1088/1361-6560/addfa6
Dosimetrically coupled multiscale tetrahedral mesh models of human liver vasculature: implications for radiopharmaceutical dosimetry of both organ blood and parenchyma
Abstract
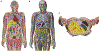
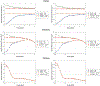
Objective.To develop a computational framework coupling multiscale vascular models of the human liver for improved radiation dosimetry calculations that clearly distinguish the absorbed dose to tissue parenchyma and that to its blood content at all spatial scales. This framework thus addresses limitations of homogeneous blood/tissue organ models in present use in radiopharmaceutical therapy.Approach.High-fidelity tetrahedral mesh models of liver vasculature were constructed at two spatial scales. At the macroscale, detailed hepatic arterial, venous, and portal venous networks were generated within reference adult male and female computational phantoms. At the microscale, a classical hexagonal liver lobule model incorporating sinusoids, bile compartments, and cellular components was developed. A mathematical framework was further developed to couple Monte Carlo radiation transport results across these spatial scales, enabling comprehensive dosimetric calculations for radiation dose to both blood and parenchymal tissues.Main Results.The coupled model system successfully accounted for the entire blood content of the liver, with approximately 31% represented in macroscale vessels (⩾100μm diameter) and 69% within microscale structures. Specific absorbed fractions were computed for monoenergetic photons, electrons, and alpha particles, demonstrating reciprocity between blood-to-parenchyma and parenchyma-to-blood crossfire. ReferenceS-values were computed for 22 therapeutic and 11 diagnostic radionuclides, providing the first comprehensive dataset for blood-specific and parenchyma-specific internal dosimetry calculations in the liver.Significance.This work establishes a novel framework for multi-scale radiation transport calculations in vascularized organs, enabling separate tracking of blood and parenchymal tissue doses. The methodology has immediate applications in improving dose calculations for radiopharmaceutical therapies, Y-90 microsphere radioembolization treatment, and analysis of blood dose during external beam radiotherapy. The approach can be readily adapted for other vascularized organs, representing a significant advancement in radiation dosimetry accuracy for both therapeutic and diagnostic applications by fully and independently accounting for organ activity localized within two tissue compartments-organ blood and organ parenchyma.
Keywords: blood self-dose; computational phantoms; liver vasculature; radiopharmaceutical dosimetry.
Creative Commons Attribution license.
Conflict of interest statement
Conflict of interest
The authors of this study report no competing interest or financial conflicts of interest.
Figures
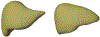
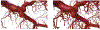








References
-
- Akabani G, Poston JW and Bolch WE 1991. Estimates of beta absorbed fractions in small tissue volumes for selected radionuclides J. Nucl. Med. 32 835–9 - PubMed
-
- Bolch WE, Eckerman KF, Sgouros G and Thomas SR 2009. MIRD pamphlet No. 21: a generalized schema for radiopharmaceutical dosimetry–standardization of nomenclature J. Nucl. Med. 50 477–84 - PubMed
-
- Boninsegna M, McCourt PAG and Holte CF 2023. The computed sinusoid Livers 3 657–73
-
- Braat MNGJA, van Erpecum KJ, Zonnenberg BA, van den Bosch MAJ and Lam MGEH 2017. Radioembolization-induced liver disease: a systematic review Eur. J. Gastroenterol. Hepatol. 29 144. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
