An open-label phase I comparator-controlled clinical trial to assess tolerability and pharmacokinetics of IHL-675 A a fixed dose combination of cannabidiol plus hydroxychloroquine in healthy volunteers
- PMID: 40456820
- PMCID: PMC12130181
- DOI: 10.1038/s41598-025-04573-5
An open-label phase I comparator-controlled clinical trial to assess tolerability and pharmacokinetics of IHL-675 A a fixed dose combination of cannabidiol plus hydroxychloroquine in healthy volunteers
Abstract
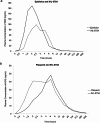
This was a phase I randomised comparator controlled clinical trial that assessed the pharmacokinetics (PK), and tolerability of IHL-675 A, a fixed dose combination (FDC) of cannabidiol (CBD) and hydroxychloroquine sulfate (HCQ), compared to the reference listed drugs Epidiolex (CBD) and Plaquenil (HCQ) in healthy volunteers (HVs). IHL-675 A is Incannex Healthcare Pty Ltd.'s proprietary product formulated using UniGel™ technology consisting of a solid, film coated HCQ tablet contained within a CBD-oil solution gel cap. Each IHL-675 A gel cap contains 75 mg of CBD and 100 mg HCQ. IHL-675 A is being developed for treatment of inflammatory conditions such as rheumatoid arthritis. This trial assessed the tolerability of IHL-675 A as well as pharmacokinetics of the active pharmaceutical ingredients CBD and HCQ as well as their major metabolites, compared to the reference listed drugs. The study included 3 treatment arms: IHL-675 A arm (150 mg CBD, 200 mg HCQ) and Plaquenil arm (200 mg HCQ) and Epidiolex (150 mg CBD) arm. Thirty-six participants were randomised into the 3 groups (12 per arm) and followed up for 4 weeks. Safety assessments including vital signs, electrocardiogram (ECG) parameters, and clinical laboratory parameters. Plasma concentrations of CBD, HCQ and their major metabolites, 7-OH-CBD, and 7-COOH-CBD, and desethylhydroxychloroquine (DHCQ), desethylchloroquine (DCQ), and bisdesethylhydroxy-chloroquine (BDCQ) were assessed at predefined timepoints across the monitoring period and PK parameters were determined and compared between treatments using noncompartmental methods and analysis of variance (ANOVA) of log-transformed values exposure PK parameters. All adverse events were coded using the current version of the Medical Dictionary for Regulatory Activities (MeDRA) by system organ class (SOC). IHL-675 A was generally well tolerated and had a similar adverse event profile compared to Epidiolex and Plaquenil. There were no SAEs reported in this study. Both CBD and HCQ were bioavailable when dosed in IHL-675 A. There were non-statistically significant differences between the pharmacokinetics of both CBD and HCQ when compared between IHL-675 A and Epidiolex or Plaquenil. There was approximately 50% increase in Cmax of CBD for IHL-675 A when compared with Epidiolex, and a slight increase of approximately 10% in AUC0-last and AUC0-inf. The 90% CI for the ratios of AUC0-inf, AUC0-last and Cmax of CBD all extended beyond the acceptance interval of 80% - 125%. The Cmax of HCQ for IHL-675 A was comparable to the reference product Plaquenil, with a geometric mean squares ratio of 96.5%. There was approximately 15% decrease in AUC0-last. The 90% CI for the ratios of AUC0-last and Cmax of HCQ extended beyond the acceptance interval of 80% - 125%. The ratio of AUC0-inf was not reliable for comparison between treatments, since the t1/2 was estimable for only 5 of 12 participants who received IHL-675 A. IHL-675 A was generally well tolerated when delivered as an oral fixed dose with no adverse events of concern or Serious Adverse Events (SAEs). Compared to the reference listed drugs for CBD (Epidiolex 150 mg) and HCQ (Plaquenil 200 mg), there was a slightly increased exposure to CBD and its metabolites for IHL-675 A, and slightly decreased exposure to HCQ. These results support the continued clinical development of IHL-675 A.Trial Registration: ACTRN12622000289718.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: GWM, PK, RW and MRB are or were employees and shareholders in Incannex Healthcare Pty Ltd. JN has no competing interests to declare that are relevant to the content of this article. Conflict of interest: GWM, PK, RW and MRB are or were employees of Incannex Healthcare Pty Ltd and have shares in the company. JN has no competing interests to declare that are relevant to the content of this article. Research involving human participants: This study was carried out according to the principles of the Declaration of Helsinki, the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) Guideline for Good Clinical Practice (GCP) E6(R2) (2016) (as adopted in Australia) and the National Health and Medical Research Council National Statement on Ethical Conduct in Human Research (2007, incorporating all updates). The study protocol was approved by Bellberry Human Research and ethics committee (123 Glen Osmond Road, Eastwood, 5063, South Australia). Informed consent: Written informed consent was obtained from each subject before any trial-related procedures were performed.
Figures
References
-
- Schrezenmeier, E. & Dörner, T. Mechanisms of action of hydroxychloroquine and chloroquine: implications for rheumatology. Nat Rev Rheumatol [Internet].16(3), 155–66. (2020). Available from: 10.1038/s41584-020-0372-x - PubMed
-
- Fox, R. I. Mechanism of action of hydroxychloroquine as an antirheumatic drug. In: Seminars in Arthritis and Rheumatism. Elsevier 82–91 (1993). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous