Spatial navigation deficits in early Alzheimer's disease: the role of biomarkers and APOE genotype
- PMID: 40456910
- PMCID: PMC12130088
- DOI: 10.1007/s00415-025-13151-8
Spatial navigation deficits in early Alzheimer's disease: the role of biomarkers and APOE genotype
Abstract
Background: Spatial navigation deficits are early symptoms of Alzheimer's disease (AD). The apolipoprotein E (APOE) ε4 allele is the most important genetic risk factor for AD. This study investigated effects of APOE genotype on spatial navigation in biomarker-defined individuals with amnestic mild cognitive impairment (aMCI) and associations of AD biomarkers and atrophy of AD-related brain regions with spatial navigation.
Methods: 107 participants, cognitively normal older adults (CN, n = 48) and aMCI individuals stratified into AD aMCI (n = 28) and non-AD aMCI (n = 31) groups, underwent cognitive assessment, brain MRI, and spatial navigation assessment using the Virtual Supermarket Test with egocentric and allocentric tasks and a self-report questionnaire. Cerebrospinal fluid (CSF) biomarkers (amyloid-β1-42, phosphorylated tau181 and total tau) and amyloid PET imaging were assessed in aMCI participants.
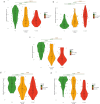
Results: AD aMCI participants had the highest prevalence of APOE ε4 carriers and worst allocentric navigation. CSF levels of AD biomarkers and atrophy in AD-related brain regions were associated with worse allocentric navigation. Between-group differences in spatial navigation and associations with AD biomarkers and regional brain atrophy were not influenced by APOE genotype. Self-reported navigation ability was similar across groups and unrelated to spatial navigation performance.
Conclusions: These findings suggest that allocentric navigation deficits in aMCI individuals are predominantly driven by AD pathology, independent of APOE genotype. This highlights the role of AD pathology as measured by biomarkers, rather than genetic status, as a major factor in navigational impairment in aMCI, and emphasizes the assessment of spatial navigation as a valuable tool for early detection of AD.
Keywords: Allocentric navigation; Amyloid-β; Egocentric navigation; Entorhinal cortex; Hippocampus; Tau protein.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflicts of interest: J.H. is a medical advisor at Neurona lab, Terrapino mobile app, consulted for Eisai, Eli Lilly, Biogen, Schwabe, and holds stock options in Alzheon. Other authors declare no competing interests. Ethical approval and consent to participate: All participants provided written informed consent according to the Declaration of Helsinki. The study was approved by the Ethics Committee of Motol University Hospital (informed consent number EK701/16).
Figures

References
-
- (2023) 2023 Alzheimer’s disease facts and figures. Alzheimers Dement 19:1598–1695. 10.1002/ALZ.13016 - PubMed
-
- Georgakas JE, Howe MD, Thompson LI et al (2023) Biomarkers of Alzheimer’s disease: past, present and future clinical use. Biomarkers Neuropsychiatry 8:100063. 10.1016/J.BIONPS.2023.100063 - DOI
MeSH terms
Substances
Grants and funding
- LX22NPO5107/National Institute for Neurological Research (Programme EXCELES, ID Project No. LX22NPO5107) - Funded by the European Union - Next Generation EU
- 00064203/Ministry of Health of the Czech Republic-conceptual development of research organization, University Hospital Motol, Prague, Czech Republic
- 6980382/Institutional Support of Excellence 3 2. LF UK
- NW25-04-00337/Ministry of Health of the Czech Republic
- 40125/Grant Agency of Charles University
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

