Outcomes of allogeneic stem cell transplant in adult Philadelphia negative acute lymphoblastic leukemia patients treated with the pediatric-inspired GIMEMA 1913 protocol. A Campus ALL study
- PMID: 40456937
- PMCID: PMC12401717
- DOI: 10.1038/s41409-025-02632-z
Outcomes of allogeneic stem cell transplant in adult Philadelphia negative acute lymphoblastic leukemia patients treated with the pediatric-inspired GIMEMA 1913 protocol. A Campus ALL study
Abstract
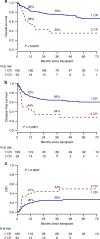
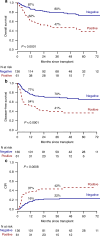
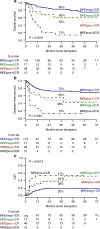
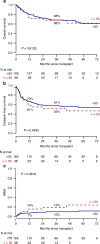
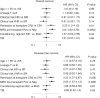
Allogeneic hematopoietic stem cell transplantation (alloHSCT) is the best consolidative treatment for high-risk acute lymphoblastic leukemia (ALL). The Campus ALL study group analyzed the clinical outcomes of patients treated in the real-life with the pediatric-inspired and minimal/measurable residual disease (MRD)-oriented GIMEMA LAL1913 protocol who underwent alloHSCT. Key factors impacting on outcomes were MRD and remission status (1st complete remission vs 2nd complete remission) at transplant. MRD positivity was associated with poorer outcomes, with 3-year overall survival (OS) and disease-free survival (DFS) of 47% and 41% in MRD-positive patients compared to 80% and 70% in MRD-negative patients. Additionally, MRD negativity was associated with improved outcomes also for patients in 2nd complete remission with 3-year OS and DFS rates of 60% and 56%, respectively, compared to only 13% for both outcomes in MRD-positive cases. Patients older than 55 years showed survival rates comparable to younger patients, despite having a slightly higher non-relapse mortality, which remained below 20% at 3 years. These findings underscore the crucial role of alloHSCT in high-risk ALL and emphasize the importance of an early accurate disease risk allocation. The adverse outcome observed with MRD positivity advocates for early pre-transplant intervention with immunotherapy, whenever possible.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures
References
-
- Boissel N, Huguet F, Leguay T, Mathilde HB, Graux C, Chalandon Y, et al. In adults with Ph-negative acute lymphoblastic leukemia (ALL), age-adapted chemotherapy intensity and MRD-driven transplant indication significantly reduces treatment-related mortality (TRM) and improves overall survival - results from the Graall-2014 Trial. Blood. 2022;140:112–4. 10.1182/blood-2022-157903. - DOI
-
- Marks DI, Kirkwood AA, Rowntree CJ, Aguiar M, Bailey KE, Beaton B, et al. Addition of four doses of rituximab to standard induction chemotherapy in adult patients with precursor B-cell acute lymphoblastic leukaemia (UKALL14): a phase 3, multicentre, randomised controlled trial. Lancet Haematol. 2022;9:e262–75. 10.1016/S2352-3026(22)00038-2. - DOI - PMC - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources

