Carbapenem-resistance in Acinetobacter baumannii: prevalence, antibiotic resistance profile and carbapenemase genes in clinical and hospital environmental strains
- PMID: 40457246
- PMCID: PMC12128347
- DOI: 10.1186/s12879-025-11169-x
Carbapenem-resistance in Acinetobacter baumannii: prevalence, antibiotic resistance profile and carbapenemase genes in clinical and hospital environmental strains
Abstract
Background: Acinetobacter baumannii, a Gram-negative member of the ESKAPE pathogen group, is known to develop resistance to several antibiotics rapidly, and carbapenem-resistant A. baumannii (CRAB) is highly implicated in life-threatening infections, especially within hospital settings.
Objectives: This study detected CRAB in clinical and hospital-environmental samples, evaluated the antibiotic resistance patterns and screened for prevalent carbapenemase genes in isolates from a hospital in Southwest Nigeria.
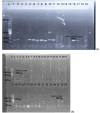
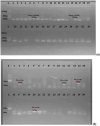
Methods: A total of 150 clinical and hospital environmental samples were analysed using culture-dependent and molecular methods for the detection of Acinetobacter baumannii. Antibiotic susceptibility test was done using the Kirby-Bauer disk diffusion technique. Phenotypic screening for carbapenemase was via simplified carbapenem inactivation method (sCIM), and molecular detection of blaKPC type, blaOXA-48-like, blaVIM type, blaNDM-1, blaIMP variants and blaOXA-23-like genes by Polymerase chain reaction.
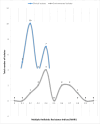
Results: Altogether, only 29.4% (42/143 isolates) of recovered isolates were identified as A. baumannii, giving a prevalence of 28.0% (42/150 samples), predominantly from sputum. All isolates had the gluconolactonase gene, while 5/42 had the blaOXA-51-like gene. Resistance to meropenem and cefiderocol was 100.0% and 88.1%, respectively, while gentamicin was most effective in vitro (7.1%); 54.8% were multidrug-resistant, and 88.1% (37/42) had MARI ≥ 0.2. Overall, 39/42 (92.9%) isolates had ≥ one or more carbapenemase genes; 61.9% (26/42) had the blaKPC type gene, 59.5% (25/42) had the blaIMP variants while 45.2% had the blaVIM type gene; no strain had the blaNDM-1 or the blaOXA-23-like gene.
Conclusion: This study reports the occurrence of MDR strains, and of blaKPC type, blaIMP variants and blaVIM type carbapenemase genes in A. baumannii isolates from clinical and hospital environmental samples, contributing to the pool of existing data on their occurrence. It also highlights the need for monitoring and continued surveillance of the strains, most especially in the clinical setting.
Keywords: bla IMP; bla KPC; bla VIM type; Carbapenem-resistant Acinetobacter baumannii; sCIM.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Ethical approval for the study was obtained from the Ethics Committee of Health Planning, Research, and Statistics Department of the Osun State Ministry of Health, Osogbo (OSHREC/PRS/569T/482), and all procedure in the study complied with the Helsinki Declaration of 1975 (in its most recently amended version). Informed consent was obtained from each participant, and parental informed consent was sought from the parents/guardians of children below 18 years. Consent for publication: Not Applicable. Competing interests: The authors declare no competing interests.
Figures



Similar articles
-
Phenotypic and molecular characterization of Acinetobacter baumannii isolates causing lower respiratory infections among ICU patients.Microb Pathog. 2019 Mar;128:75-81. doi: 10.1016/j.micpath.2018.12.023. Epub 2018 Dec 15. Microb Pathog. 2019. PMID: 30562602
-
Outbreak of carbapenem-resistant Acinetobacter baumannii carrying the carbapenemase OXA-23 in ICU of the eastern Heilongjiang Province, China.BMC Infect Dis. 2019 May 22;19(1):452. doi: 10.1186/s12879-019-4073-5. BMC Infect Dis. 2019. PMID: 31113374 Free PMC article.
-
A prospective multicenter study on the evaluation of antimicrobial resistance and molecular epidemiology of multidrug-resistant Acinetobacter baumannii infections in intensive care units with clinical and environmental features.Ann Clin Microbiol Antimicrob. 2019 Jul 2;18(1):19. doi: 10.1186/s12941-019-0319-8. Ann Clin Microbiol Antimicrob. 2019. PMID: 31266519 Free PMC article.
-
Identification of blaOXA-51-23-58, blaVIM, blaNDM, and blaIMP carbapenemase genes in Acinetobacter baumannii isolates from hospitalized patients.BMC Res Notes. 2024 Dec 27;17(1):392. doi: 10.1186/s13104-024-07047-5. BMC Res Notes. 2024. PMID: 39736661 Free PMC article.
-
Prevalence of different carbapenemase genes among carbapenem-resistant Acinetobacter baumannii blood isolates in Taiwan.Antimicrob Resist Infect Control. 2018 Oct 11;7:123. doi: 10.1186/s13756-018-0410-5. eCollection 2018. Antimicrob Resist Infect Control. 2018. PMID: 30338061 Free PMC article.
References
-
- Whiteway C, Breine A, Philippe C, Van der Henst C. Acinetobacter baumannii. Trends Microbiol Febr. 2022;30(2). 10.1016/j.tim.2021.11.008 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

