In situ radiochemical doping of functionalized inorganic nanoplatforms for theranostic applications: a paradigm shift in nanooncology
- PMID: 40457312
- PMCID: PMC12128498
- DOI: 10.1186/s12951-025-03472-1
In situ radiochemical doping of functionalized inorganic nanoplatforms for theranostic applications: a paradigm shift in nanooncology
Abstract
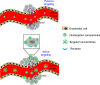
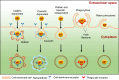
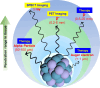
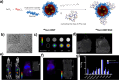
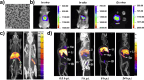
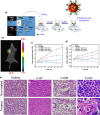
In situ radiochemical doping presents a transformative approach for synthesizing radiolabeled inorganic nanoparticles (NPs) for cancer theranostics. Traditional radiolabeling techniques rely on bifunctional chelators, which often require harsh reaction conditions that can degrade the physicochemical properties of NPs. Additionally, the enzymatic dissociation of radiometals can potentially induce in vivo toxicity. In contrast, in situ doping directly incorporates radiometals into the NP crystal lattice, significantly enhancing both radiolabeling yield and radiochemical stability. This method preserves the pharmacokinetic profiles of the radiolabeled NPs, improving their theranostic efficacy. This review provides an up-to-date overview of the progress made in the development of radiolabeled inorganic nanoplatforms through in situ doping, with a focus on their stability, physicochemical characteristics, and applications in cancer theranostics. Our findings highlight the advantages in situ doping as a more efficient and stable alternative to conventional radiolabeling methods, offering substantial potential for the development of more effective cancer theranostic agents.
Keywords: Cancer; Doping; In situ; Nanoparticles; Precision oncology; Radiation therapy; Radiolabeling; Theranostics.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: Weibo Cai declares conflict of interest with the following corporations: Portrai, Inc., rTR Technovation Corporation, and Four Health Global Pharmaceuticals Inc. All other authors declare no competing of interest.
Figures











References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

