Mitochondrial dynamics reveal potential to facilitate axonal regeneration after spinal cord injury
- PMID: 40457377
- PMCID: PMC12131810
- DOI: 10.1186/s12967-025-06611-2
Mitochondrial dynamics reveal potential to facilitate axonal regeneration after spinal cord injury
Abstract
Background: Spinal cord injury (SCI) arises from traumatic damage to the spinal cord, resulting in varying degrees of sensory, motor, and autonomic dysfunction. Mitochondria, as the primary energy-producing organelles within cells, have garnered increasing attention for their critical role in promoting axonal regeneration following SCI.
Aim of review: This review aims to systematically examine the alterations in mitochondrial dynamics post-SCI and to elucidate their influence on axonal regeneration. Furthermore, the review evaluates the current challenges associated with SCI treatment and proposes potential therapeutic strategies for future research.
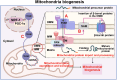
Key scientific concepts of review: The review comprehensively addresses mitochondrial dynamics, with a focus on key processes such as biogenesis, fusion and fission, mitophagy, trafficking, and anchoring. It delves into the molecular mechanisms by which signaling pathways within neurons and glial cells regulate these mitochondrial processes to facilitate axonal regeneration. Additionally, the review identifies existing challenges in SCI treatment and advocates for targeted interventions in mitochondrial dynamics as a promising therapeutic avenue, offering significant potential for advancing future research and treatment of SCI.
Keywords: Axonal mitochondria; Axonal regeneration; Dynamics; Spinal cord injury.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Consent for publication: Not applicable. Ethics approval: Not applicable. Competing interests: The authors declare that they have no competing interests.
Figures





Similar articles
-
Mechanism and prospects of mitochondrial transplantation for spinal cord injury treatment.Stem Cell Res Ther. 2024 Nov 28;15(1):457. doi: 10.1186/s13287-024-04077-5. Stem Cell Res Ther. 2024. PMID: 39609871 Free PMC article. Review.
-
The role of mitochondrial energy metabolism in neuroprotection and axonal regeneration after spinal cord injury.Mitochondrion. 2023 Mar;69:57-63. doi: 10.1016/j.mito.2023.01.009. Epub 2023 Feb 4. Mitochondrion. 2023. PMID: 36740158 Review.
-
Regulation of axonal regeneration following spinal cord injury in the lamprey.J Neurophysiol. 2017 Sep 1;118(3):1439-1456. doi: 10.1152/jn.00986.2016. Epub 2017 May 3. J Neurophysiol. 2017. PMID: 28469003 Free PMC article.
-
Bone marrow mesenchymal stem cells (BMSCs) improved functional recovery of spinal cord injury partly by promoting axonal regeneration.Neurochem Int. 2018 May;115:80-84. doi: 10.1016/j.neuint.2018.02.007. Epub 2018 Feb 16. Neurochem Int. 2018. PMID: 29458076 Review.
-
Glial and axonal regeneration following spinal cord injury.Cell Adh Migr. 2009 Jan-Mar;3(1):99-106. doi: 10.4161/cam.3.1.7372. Epub 2009 Jan 7. Cell Adh Migr. 2009. PMID: 19372750 Free PMC article. Review.
References
Publication types
MeSH terms
Grants and funding
- Jiangsu science and education of traditional Chinese medicine〔2021〕No. 4/Supported by the Foundation of Jiangsu CM Clinical Innovation Center of Degenerative Bone & Joint Disease
- NATCM's Human Education Letter[2023]No. 85/NATCM's Project of High-level Construction of Key TCM Disciplines
- 82204934/National Natural Science Foundation of China
- 81973885/National Natural Science Foundation of China
- QN202001/Jiangsu Chinese Medicine Science and Technology Development Project
LinkOut - more resources
Full Text Sources
Medical
Research Materials

