Two unique cases of eosinophilic granulomatosis with polyangiitis in childhood treated with anti-interleukin-5 therapy: infantile-onset and submandibular salivary gland involvement
- PMID: 40457436
- PMCID: PMC12128228
- DOI: 10.1186/s12969-025-01115-1
Two unique cases of eosinophilic granulomatosis with polyangiitis in childhood treated with anti-interleukin-5 therapy: infantile-onset and submandibular salivary gland involvement
Abstract
Background: ANCA-associated vasculitis is a systemic autoimmune disease involving small- and medium-sized blood vessels. Eosinophilic granulomatosis with polyangiitis (EGPA, previously Churg Strauss Syndrome) is the least common form in childhood with few cases reported. We present two unique pediatric cases, both of which were treated with anti-interleukin-5 therapy.
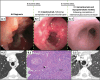
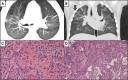
Case presentation: Case one is a 13-year-old male with asthma and allergies who presented with one month of cough and periorbital edema and subsequently developed submandibular swelling. Evaluation identified chronic sinusitis, weight loss, positive c-ANCA and anti-MPO IgG antibodies, peripheral blood eosinophilia, pulmonary eosinophilia, tracheal and pulmonary nodules, and eosinophilic infiltration of the submandibular salivary gland with granulomas and fibrosis fitting a diagnosis of EGPA. He improved with glucocorticoids and mepolizumab with a significant partial response, and eventually switched to benralizumab and mycophenolate mofetil with complete response. Case two presented at 19-months-old in acute respiratory distress with a history of reactive airway disease. EGPA diagnosis was confirmed on lung biopsy (eosinophilic capillaritis and interstitial expansion of eosinophils) in the setting of anti-MPO and p-ANCA positivity. He has done very well on mepolizumab for three years.
Conclusions: To our knowledge, this is the first reported case of submandibular salivary gland infiltrate in a child with EGPA and the youngest successfully treated patient with EGPA reported in the literature. These cases demonstrate the variation in age and disease manifestations seen in children with EGPA as well as positive responses to anti-interleukin-5 therapy. Children with EGPA may present with common or unusual complaints and require astute recognition to avoid delays in diagnosis and long-term damage.
Keywords: ANCA-associated vasculitis; Benralizumab; Churg Strauss; EGPA; Eosinophilic granulomatosis with polyangiitis; Mepolizumab; Sialadenitis; anti-IL5 therapy.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Consent for publication: Written consent was obtained from both patients. Competing interests: The authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

