Short-Term DMOG treatment rejuvenates senescent mesenchymal stem cells by enhancing mitochondrial function and mitophagy through the HIF-1α/BNIP3 pathway
- PMID: 40457488
- PMCID: PMC12131571
- DOI: 10.1186/s13287-025-04422-2
Short-Term DMOG treatment rejuvenates senescent mesenchymal stem cells by enhancing mitochondrial function and mitophagy through the HIF-1α/BNIP3 pathway
Abstract
Background: Mesenchymal stem cells (MSCs) have potential for treating degenerative and immune diseases, but their clinical efficacy is limited by senescence, characterized by mitochondrial dysfunction, impaired mitophagy, and metabolic imbalance. The goal of this study was to investigate the effects of dimethyloxalylglycine (DMOG), a hypoxia-mimetic agent that stabilizes hypoxia-inducible factor 1 alpha (HIF-1α), on rejuvenating senescent MSCs by enhancing mitochondrial function, mitophagy, and metabolic reprogramming.
Methods: Two models of MSC senescence were established: oxidative stress-induced senescence using hydrogen peroxide and replicative senescence through serial passaging. Umbilical cord derived MSCs were treated with DMOG for 48 h under normoxic conditions. Mitochondrial function, mitophagy, and metabolism were assessed using assays that measured mitochondrial membrane potential, reactive oxygen species levels, ATP production, and mitophagy. Western blotting and real-time PCR were employed to analyze the expression changes of relevant molecules. RNA sequencing (RNA-seq) was performed to identify key genes and pathways regulated by DMOG. Additionally, to evaluate the therapeutic potential of rejuvenated MSCs, a co-culture system was established, where DMOG-treated senescent MSCs were co-cultured with IL-1β-treated chondrocytes.
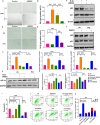
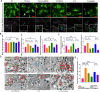
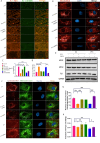
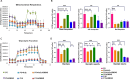
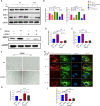
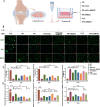
Results: DMOG treatment significantly reduced key senescence markers, including senescence-associated beta-galactosidase, p53, and p21, in both senescence models. DMOG treatment restored mitochondrial morphology and function, improving mitochondrial membrane potential, reducing mitochondrial reactive oxygen species, and enhancing ATP production. DMOG also promoted mitophagy, as evidenced by increased colocalization of mitochondria with lysosomes. RNA-seq analysis revealed that DMOG activated key pathways, including HIF-1 signaling, calcium signaling, and mitophagy-related gene (BNIP3 and BNIP3L). Notably, BNIP3 knockdown greatly abolished DMOG-induced mitophagy and its anti-senescence effects. Furthermore, DMOG treatment improved metabolic flexibility by enhancing both mitochondrial respiration and glycolysis in senescent MSCs. Moreover, DMOG-treated senescent MSCs partially restored their therapeutic efficacy in an osteoarthritis model by improving extracellular matrix regulation in IL-1β-stimulated chondrocytes.
Conclusions: Short-term DMOG treatment rejuvenates senescent MSCs by enhancing mitochondrial function, promoting mitophagy via HIF-1α/BNIP3, and improving metabolic reprogramming. DMOG-treated MSCs also showed enhanced therapeutic efficacy in co-culture with IL-1β-treated chondrocytes, suggesting its potential to improve MSC-based therapies in regenerative medicine.
Keywords: BNIP3; HIF-1α; Hypoxia-Mimetic agent; Mesenchymal stem cells (MSCs); Mitophagy; Senescence.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethical approval: The ethics Committee of Chinese PLA General Hospital in China approved the isolation of chondrocytes from articular cartilage samples (Beijing, China). (1) Title of the approved project: Study on the treatment of cartilage cell mitochondrial damage in aging MSCs through hypoxic preconditioning. (2) Name of the institutional approval committee or unit: The Clinical Trial Ethics Committee of the Fourth Medical Center of the PLA General Hospital. 3.Approval number: 2024KY0132-KS001. 4. Date of approval: October 8, 2024. Consent for publication: Not applicable. Declaration of AI assisted copy editing in the writing process: During the preparation of this work the author(s) used ChatGPT to improve the language. After using this tool, the authors reviewed and edited the content as required and took full responsibility for the content of the publication. Competing of interest: The authors declare that they have no competing interests.
Figures

Similar articles
-
Stabilization of HIF-1α alleviates osteoarthritis via enhancing mitophagy.Cell Death Dis. 2020 Jun 25;11(6):481. doi: 10.1038/s41419-020-2680-0. Cell Death Dis. 2020. PMID: 32587244 Free PMC article.
-
Melatonin suppresses senescence-derived mitochondrial dysfunction in mesenchymal stem cells via the HSPA1L-mitophagy pathway.Aging Cell. 2020 Mar;19(3):e13111. doi: 10.1111/acel.13111. Epub 2020 Jan 22. Aging Cell. 2020. PMID: 31965731 Free PMC article.
-
The role of HIF-1α/BNIP3/mitophagy in acrylonitrile-induced neuronal death in HT22 cells and mice: A potential neuroprotection target.Chem Biol Interact. 2025 Jan 25;406:111327. doi: 10.1016/j.cbi.2024.111327. Epub 2024 Nov 29. Chem Biol Interact. 2025. PMID: 39615733
-
A Mesenchymal stem cell Aging Framework, from Mechanisms to Strategies.Stem Cell Rev Rep. 2024 Aug;20(6):1420-1440. doi: 10.1007/s12015-024-10732-4. Epub 2024 May 10. Stem Cell Rev Rep. 2024. PMID: 38727878 Review.
-
The critical role of mitophagy in cell senescence-mediated pulmonary fibrosis and potential therapeutic strategies.Mol Biol Rep. 2025 Jun 7;52(1):565. doi: 10.1007/s11033-025-10665-2. Mol Biol Rep. 2025. PMID: 40481901 Review.
References
-
- Swain HN, Boyce PD, Bromet BA, Barozinksy K, Hance L, Shields D, Olbricht GR, Semon JA. Mesenchymal stem cells in autoimmune disease: A systematic review and meta-analysis of pre-clinical studies. Biochimie. 2024;223:54–73. - PubMed
-
- Wang G, Xing D, Liu W, Zhu Y, Liu H, Yan L, Fan K, Liu P, Yu B, Li JJ, Wang B. Preclinical studies and clinical trials on mesenchymal stem cell therapy for knee osteoarthritis: A systematic review on models and cell doses. Int J Rheum Dis. 2022;25:532–62. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

