A genome-wide RNA interference screening reveals protectiveness of SNX5 knockdown in a Parkinson's disease cell model
- PMID: 40457499
- PMCID: PMC12131658
- DOI: 10.1186/s40035-025-00486-5
A genome-wide RNA interference screening reveals protectiveness of SNX5 knockdown in a Parkinson's disease cell model
Abstract
Background: Alpha-synuclein (αSyn) is a major player in the pathophysiology of synucleinopathies, which include Parkinson's disease, dementia with Lewy bodies, and multiple system atrophy. To date, there is no disease-modifying therapy available for these synucleinopathies. Furthermore, the intracellular mechanisms by which αSyn confers toxicity are not yet fully understood. Therefore, it is of utmost importance to investigate the pathophysiology of αSyn-induced toxicity in order to identify novel molecular targets for the development of disease-modifying therapies.
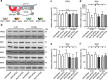
Methods: We performed the first genome-wide siRNA modifier screening in a human postmitotic neuronal cell model using αSyn-induced toxicity as a read-out. In a multi-step approach, we identified several genes, whose knockdown protected against αSyn-induced toxicity. The main hit was further validated by different methods, including immunofluorescence microscopy, qPCR, and Western blot. Furthermore, the main finding was confirmed in mouse primary neurons.
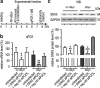
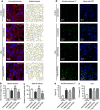
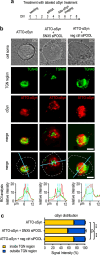
Results: The highest protection was achieved by knockdown of SNX5, which encodes the sorting nexin 5 (SNX5) protein, a component of the retromer complex. The protective efficacy of SNX5 knockdown was confirmed with an independent siRNA system. The protective effect of SNX5 knockdown was further confirmed in primary neurons from transgenic mice, where the knockdown of SNX5 led to amelioration of decrease in synchrony that was observed in untreated and control-siRNA-treated cells. SNX5 protein is a component of the SNX-BAR (Bin/Amphiphysin/Rvs) heterodimer, which is part of the retromer complex. Extracellular αSyn and overexpression of intracellular αSyn led to fragmentation of the trans-Golgi network, which was prevented by SNX5 knockdown that led to confinement of αSyn in early endosomes.
Conclusion: In summary, our data suggest that SNX5 plays an important role in the trafficking and toxicity of αSyn. Therefore, SNX5 appears to be a target of therapeutic intervention for synucleinopathies.
Keywords: Alpha-synuclein; Genome-wide RNAi screening; Parkinson’s disease; Retromer; SNX5; Trans-Golgi network.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Consent for publication: Not applicable. Competing interests: All authors have no competing interests.
Figures





References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

