Differentiation Defect Into GABAergic Neurons in Cerebral Organoids From Autism Patients
- PMID: 40457513
- PMCID: PMC12129711
- DOI: 10.1111/cns.70449
Differentiation Defect Into GABAergic Neurons in Cerebral Organoids From Autism Patients
Abstract
Objectives: Autism spectrum disorder (ASD) is a neurodevelopmental condition that affects social communication and behaviors. While previous studies using animal models have substantially expanded our knowledge about ASD, the lack of an appropriate human model system that accurately recapitulates the human-specific pathophysiology of ASD hinders the precise understanding of its etiology and the development of effective therapies. This study aims to replicate pathological phenotypes in cerebral organoids derived from idiopathic ASD patients and to conduct proof-of-concept research for the development of ASD therapeutics.
Methods: We conducted an in vitro disease modeling study using cerebral organoids derived from three idiopathic ASD patients. Additionally, we performed organoid-based phenotypic drug screening to identify potential therapeutic compounds that could ameliorate the phenotypes observed in cerebral organoids derived from idiopathic ASD patients.
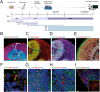
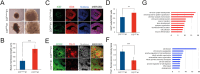
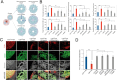
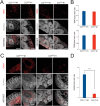
Results: Here we show that cerebral organoids derived from idiopathic ASD patients display malformation of the ventricular zones and impaired early neuronal differentiation. Through organoid-based phenotypic drug screening, we successfully generated cerebral organoids with normal tissue architecture in which the delayed neuronal differentiation could also be accelerated. Notably, cerebral organoids from ASD patients exhibited a reduced number of GABAergic neurons compared to healthy controls, resulting in an imbalance in the excitatory and inhibitory neuron ratio. The differentiation defects into GABAergic neurons in patient-derived cerebral organoids could be rescued by treating with either IGF1 or Gabapentin, a GABA agonist.
Conclusions: Our findings provide a framework for utilizing patient-derived cerebral organoids in the development of personalized pharmaceutical treatment for ASD.
Keywords: autism spectrum disorder; cerebral organoids; disease modeling; drug screening.
© 2025 The Author(s). CNS Neuroscience & Therapeutics published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- AlSalehi S. M. and Alhifthy E. H., “Autism Spectrum Disorder. Clinical Child ,” Neurology (2020): 275–292.
-
- Marshall J. J. and Mason J. O., “Mouse vs. Man: Organoid Models of Brain Development & Disease,” Brain Research 1724 (2019): 146427. - PubMed
MeSH terms
Grants and funding
- 2021R1A2C1091893/National Research Foundation of Korea
- Priority Academic Program Development of Jiangsu Higher Education Institutions
- RS-2022-00090257/Ministry of Health and Welfare of South Korea
- 31971319/National Natural Science Foundation of China
- 32171403/National Natural Science Foundation of China
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

