Intra-Arterial Deoxyribonuclease Therapy Improves Stroke Outcomes in Aged Mice
- PMID: 40457526
- PMCID: PMC12129710
- DOI: 10.1111/cns.70461
Intra-Arterial Deoxyribonuclease Therapy Improves Stroke Outcomes in Aged Mice
Abstract
Background: Futile recanalization affects more than half of acute ischemic stroke (AIS) patients. Neutrophil extracellular traps (NETs) are a major factor of microvascular hypoperfusion after stroke. Deoxyribonuclease I (DNase) targeting NETs exhibited a neuroprotective effect in young mice with AIS. This study explored a novel direct intra-arterial administration of DNase therapy and its effect in aged mice with AIS.
Method: AIS was induced in aged C57BL/6 mice followed by reperfusion and immediate, intra-arterial DNase administration via the internal carotid artery. Cerebral blood flow (CBF), neurological function, cerebral infarct volume, and NET markers were examined.
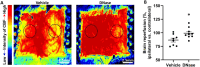
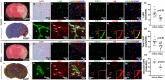
Results: Direct intra-arterial DNase therapy significantly increased CBF, reduced neurological deficit scores, increased the latency to fall in the wire hang test, reduced cerebral infarct volume, and decreased neutrophil and NET count in both the parenchyma and micro vessels in aged mice with AIS compared with age-matched vehicle controls.
Conclusion: Our data is the first to demonstrate that successful, direct intra-arterial DNase therapy provides more efficient cerebral reperfusion and better outcomes after recanalization during the treatment of large vessel occlusion in aged mice. This study provides evidence for the potential clinical application of catheter-delivered intra-arterial DNase therapy post-recanalization.
Keywords: acute ischemic stroke; deoxyribonuclease; futile recanalization; intra‐arterial therapy; neutrophil; neutrophil extracellular traps.
© 2025 The Author(s). CNS Neuroscience & Therapeutics published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors have nothing to report.
The authors declare no conflicts of interest.
Figures



References
-
- DALYs GBD and Collaborators H , “Global, Regional, and National Disability‐Adjusted Life‐Years (DALYs) for 359 Diseases and Injuries and Healthy Life Expectancy (HALE) for 195 Countries and Territories, 1990–2017: A Systematic Analysis for the Global Burden of Disease Study 2017,” Lancet 392 (2018): 1859–1922. - PMC - PubMed
-
- Lattanzi S., Cuccurullo C., Orlandi N., et al., “Futile Recanalization Is Associated With Increased Risk of Post‐Stroke Epilepsy,” Journal of the Neurological Sciences 462 (2024): 123067. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

