Hippo pathway effectors are associated with glioma patient survival, control cell proliferation and sterol metabolism through TEAD3
- PMID: 40457844
- PMCID: PMC12488254
- DOI: 10.1111/bpa.70021
Hippo pathway effectors are associated with glioma patient survival, control cell proliferation and sterol metabolism through TEAD3
Abstract
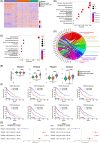
Glioblastomas represent the most common and lethal primary brain tumors in the world. Despite therapeutic advances during the last two decades, patient prognosis remains very poor. The Hippo signaling pathway effectors YAP/TAZ-TEADs play a crucial role in tumor progression and represent promising therapeutic targets in gliomas. In this study, we identified and investigated the clinical and biological significance of TEAD transcription factors. Through comprehensive analyses of TCGA glioma data and patient samples, we identified TEAD3-4 transcription factors as robust prognostic markers of patient outcome. Using up to five different patient-derived glioblastoma stem cell cultures, we confirmed the preferential expression and activation of TEAD3-4 along with their transcriptional coactivators YAP/TAZ. Pharmacological inhibition of YAP/TAZ-TEAD interaction by Verteporfin significantly decreased tumor cell growth, whereas specific inhibition of TEAD3 did not impact cell proliferation but affected sterol/cholesterol biosynthetic and metabolic processes. This study contributes to a better understanding of the role of Hippo effectors in glioblastoma pathophysiology. These transcription factors, particularly TEAD3, could potentially serve as therapeutic targets, especially considering recent data on cholesterol homeostasis in glioblastomas.
Keywords: TEAD3; glioblastomas; gliomas; hippo pathway; sterol metabolism.
© 2025 The Author(s). Brain Pathology published by John Wiley & Sons Ltd on behalf of International Society of Neuropathology.
Conflict of interest statement
The authors have no conflicts of interest to disclose.
Figures



References
-
- Ostrom QT, Gittleman H, Stetson L, Virk SM, Barnholtz‐Sloan JS. Epidemiology of gliomas. Cancer Treat Res. 2015;163:1–14. - PubMed
-
- van den Bent MJ, Geurts M, French PJ, Smits M, Capper D, Bromberg JEC, et al. Primary brain tumours in adults. Lancet. 2023;402:1564–1579. - PubMed
-
- Huang J, Wu S, Barrera J, Matthews K, Pan D. The hippo signaling pathway coordinately regulates cell proliferation and apoptosis by inactivating Yorkie, the drosophila homolog of YAP. Cell. 2005;122:421–434. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

