Oilseed-based metabolic engineering of astaxanthin and related ketocarotenoids using a plant-derived pathway: Lab-to-field-to-application
- PMID: 40457958
- PMCID: PMC12310876
- DOI: 10.1111/pbi.70148
Oilseed-based metabolic engineering of astaxanthin and related ketocarotenoids using a plant-derived pathway: Lab-to-field-to-application
Abstract
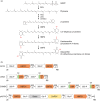
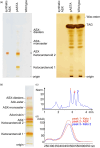
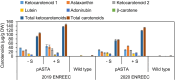
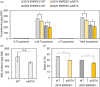
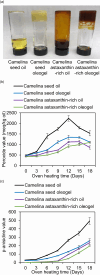
Ketocarotenoids, including astaxanthin, are red lipophilic pigments derived from the oxygenation of β-carotene ionone rings. These carotenoids have exceptional antioxidant capacity and high commercial value as natural pigments, especially for aquaculture feedstocks to confer red flesh colour to salmon and shrimp. Ketocarotenoid biosynthetic pathways occur only in selected bacterial, algal, fungal and plant species, which provide genetic resources for biotechnological ketocarotenoid production. Toward pathway optimization, we developed a transient platform for ketocarotenoid production using Agrobacterium infiltration of Nicotiana benthamiana leaves with plant (Adonis aestivalis) genes, carotenoid β-ring 4-dehydrogenase 2 (CBFD2) and carotenoid 4-hydroxy-β-ring 4-dehydrogenase (HBFD1), or bacterial (Brevundimonas) genes, β-carotene ketolase (crtW) and β-carotene hydroxylase (crtZ). In this test system, heterologous expression of the plant-derived astaxanthin pathway conferred higher astaxanthin production with fewer ketocarotenoid intermediates than the bacterial pathway. We evaluated the plant-derived pathway for ketocarotenoid production using the oilseed camelina (Camelina sativa) as a production platform. Genes for CBFD2 and HBFD1 and maize phytoene synthase were introduced under the control of seed-specific promoters. In contrast to prior research with bacterial pathways, our strategy resulted in nearly complete conversion of β-carotene to ketocarotenoids, including primarily astaxanthin. Tentative identities of other ketocarotenoids were established by chemical evaluation. Seeds from multi-season US and UK field sites maximally accumulated ~135 μg/g seed weight of ketocarotenoids, including astaxanthin (~47 μg/g seed weight). Although plants had no observable growth reduction, seed size and oil content were reduced in astaxanthin-producing lines. Oil extracted from ketocarotenoid-accumulating seeds showed significantly enhanced oxidative stability and was useful for food oleogel applications.
Keywords: antioxidant; aquaculture; camelina; β‐carotene hydroxylase; β‐carotene ketolase.
© 2025 The Author(s). Plant Biotechnology Journal published by Society for Experimental Biology and The Association of Applied Biologists and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no competing financial interests.
Figures


References
-
- Alcaíno, J. , Fuentealba, M. , Cabrera, R. , Baeza, M. and Cifuentes, V. (2012) Modeling the interfacial interactions between CrtS and CrtR from Xanthophyllomyces dendrorhous, a P450 system involved in astaxanthin production. J. Agric. Food Chem. 60, 8640–8647. - PubMed
-
- Allorent, G. , Osorio, S. , Ly Vu, J. , Falconet, D. , Jouhet, J. , Kuntz, M. , Fernie, A.R. et al. (2014) Adjustments of embryonic photosynthetic activity modulate seed fitness in Arabidopsis thaliana . New Phytologist 205(2), 707–719. - PubMed
-
- Álvarez, V. , Rodríguez‐Sáiz, M. , de la Fuente, J.L. , Gudiña, E.J. , Godio, R.P. , Martín, J.F. and Barredo, J.L. (2006) The crtS gene of Xanthophyllomyces dendrorhous encodes a novel cytochrome‐P450 hydroxylase involved in the conversion of β‐carotene into astaxanthin and other xanthophylls. Fungal Genet. Biol. 43, 261–272. - PubMed
MeSH terms
Substances
Grants and funding
- 2435264/National Science Foundation
- 2021-67013-33899;2022-67037-36616/U.S. Department of Agriculture, National Institute of Food and Agriculture
- Nebraska Soybean Board
- BBS/E/C/000I0420/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BBS/E/RH/23002B/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Miscellaneous

