Building on the Translational Science Benefits Model to include team science: a practical and theory-based approach to continuous quality improvement and impact evaluation for Clinical and Translational Science Award programs
- PMID: 40458088
- PMCID: PMC12127396
- DOI: 10.3389/fpubh.2025.1581205
Building on the Translational Science Benefits Model to include team science: a practical and theory-based approach to continuous quality improvement and impact evaluation for Clinical and Translational Science Award programs
Abstract
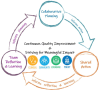
Introduction: Clinical and Translational Science Award (CTSA) programs seek to improve the quality and impact of clinical and translational science. CTSA evaluation teams implement structured, evidence-based continuous quality improvement (CQI) processes to enhance activities and outcomes, ultimately benefiting public health. The Translational Science Benefits Model (TSBM) provides a framework for assessing translational science's health and societal impact, yet additional tools are needed to integrate CQI with impact evaluation. Addressing this gap requires combining CQI methodologies with team science approaches. Building on TSBM, CQI theories (e.g., Plan-Do-Study-Act cycles), and team science principles (e.g., inclusive leadership), we propose a theory-driven, evidence-based logic model to enhance CTSA programs. Using our TL1 Regenerative Medicine Training Program (RMTP) as a case study, we demonstrate its practical application for CTSA evaluation teams.
Methods: We conducted a literature review on impact evaluation, CQI, and team science to develop a theory-based approach for CTSA evaluation teams. Using case study methodology, we analyzed RMTP data (2015-2023) through: (a) Interviews with RMTP leaders, mentors, and trainees to explore program implementation and outcomes; (b) Document analysis of program materials, meeting notes, and reports; (c) Bibliometric and policy analysis of publications, citations, and policy documents to assess impact; and (d) Surveys to capture trainees' perspectives on program quality and leadership. This mixed-methods approach provided a comprehensive assessment of RMTP's impact and demonstrated the utility of our team science-based approach to CQI and evaluation.
Results: Our sample included RMTP directors (N = 2), mentors (N = 24), and trainees (N = 38). Among trainees, 68% identified as female, and 21% were from underrepresented groups in medicine. Of 34 graduates, 31 continued in regenerative medicine research. Qualitative data highlighted CQI strategies, such as embedding evaluation into advisory meetings to enhance program functioning. Inclusive leadership fostered a climate where diverse perspectives informed improvements. Quantitative and document analysis further demonstrated how RMTP activities led to positive health and societal impacts within the TSBM framework.
Discussion: CTSA evaluation teams must integrate CQI and impact evaluation, yet few theory-based approaches exist. Our evaluation and CQI framework merges TSBM, CQI, and team science principles, providing a practical tool for guiding evaluation teams in continuous improvement while maximizing translational science impact.
Keywords: Clinical and Translational Science Award; Translational Science Benefits Model; continuous quality improvement; evaluation; logic models.
Copyright © 2025 Brimhall, Kuhfeldt, Kotton and Jones.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Department of Health and Human Services . PAR-24-272: Clinical and Translational Science Award (UM1 Clinical Trial Optional). NIH. Available online at: https://grants.nih.gov/grants/guide/pa-files/PAR-24-272.html (accessed February 6, 2025).
-
- Luke DA, Sarli CC, Suiter AM, Carothers BJ, Combs TB, Allen JL, et al. The translational science benefits model: a new framework for assessing the health and societal benefits of clinical and translational sciences. Clin Transl Sci. (2018) 11:77–84. doi: 10.1111/CTS.12495, PMID: - DOI - PMC - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

