Monoclonal antibodies targeting Candida disrupt biofilms and inhibit growth across global clinical isolates
- PMID: 40458186
- PMCID: PMC12127592
- DOI: 10.1016/j.isci.2025.112459
Monoclonal antibodies targeting Candida disrupt biofilms and inhibit growth across global clinical isolates
Abstract
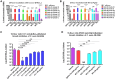
Candida species are a major cause of severe fungal infections, particularly in immunocompromised individuals and hospitalized patients. Among these, Candida auris stands out as an emerging "superbug", responsible for life-threatening bloodstream infections with mortality rates up to 60%. This multidrug-resistant, hospital-acquired pathogen has been declared a serious global health threat, complicating effective treatment and increasing mortality risks. Our research has identified universal monoclonal antibodies (mAbs) targeting Candida albicans cell wall epitopes, which are highly homologous to those in other pathogenic Candida species, including C. auris. Passive transfer of mAbs-C3.1 (anti-β-1,2-mannotriose) and 9F2 (anti-phosphoglycerate kinase 1)-significantly extended survival and improved fungal clearance in a complement C5-deficient A/J murine model. Additionally, C3.1 and 9F2 mAbs show direct fungicidal effects against C. auris isolates, presenting a promising therapeutic option against this critical threat.
Keywords: Immunology; Medical microbiology; Mycology.
© 2025 The Authors.
Conflict of interest statement
US Patent Application No. 18/143,046 Entitled: VACCINE COMPOSITIONS AND METHODS OF USING THE SAME Inventors: XIN, Hong Filed: May 03, 2023 HSCNO Ref. No.: HSCNO-2014-33-04 Our Ref. No.: 2932719-000012-US3 Response to formalities notice due: February 11, 2024 (not extendable)
Figures





References
-
- Armstrong P.A., Rivera S.M., Escandon P., Caceres D.H., Chow N., Stuckey M.J., Díaz J., Gomez A., Vélez N., Espinosa-Bode A., et al. Hospital-associated multicenter outbreak of emerging fungus Candida auris, Colombia, 2016. Emerg. Infect. Dis. 2019;25:1339–1346. doi: 10.3201/eid2507.180491. - DOI - PMC - PubMed
-
- Lockhart S.R., Etienne K.A., Vallabhaneni S., Farooqi J., Chowdhary A., Govender N.P., Colombo A.L., Calvo B., Cuomo C.A., Desjardins C.A., et al. Simultaneous Emergence of Multidrug-Resistant Candida auris on 3 Continents Confirmed by Whole-Genome Sequencing and Epidemiological Analyses. Clin. Infect. Dis. 2017;64:134–140. doi: 10.1093/cid/ciw691. - DOI - PMC - PubMed
-
- Parra-Giraldo C.M., Valderrama S.L., Cortes-Fraile G., Garzón J.R., Ariza B.E., Morio F., Linares-Linares M.Y., Ceballos-Garzón A., de la Hoz A., Hernandez C., et al. First report of sporadic cases of Candida auris in Colombia. Int. J. Infect. Dis. 2018;69:63–67. doi: 10.1016/j.ijid.2018.01.034. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

