ANKRD17 induces pro-survival signaling pathways that enhance cellular invasion and migration during hepatocellular carcinoma tumorigenesis
- PMID: 40458187
- PMCID: PMC12127597
- DOI: 10.1016/j.isci.2025.112463
ANKRD17 induces pro-survival signaling pathways that enhance cellular invasion and migration during hepatocellular carcinoma tumorigenesis
Abstract
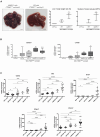
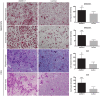
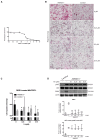
Metastasis is the primary cause of high mortality in patients with hepatocellular carcinoma (HCC) . A prior study identified ankyrin repeat domain 17 (Ankrd17) as a key gene linked to HCC metastasis. Through reverse genetics, it was observed that mouse liver tumors overexpressing ANKRD17 exhibited a higher tumor load and increased expression of endothelial-mesenchymal transition (EMT) markers. Similarly, ANKRD17 overexpression in human liver cell lines resulted in an amplified cellular motility and invasion capability, whereas knockdown studies reversed this effect. Abnormal regulation of signaling pathways was linked to increased metastasis and survival in cells overexpressing ANKRD17. Notably, the pro-metastatic discoidin domain receptor tyrosine kinase 1 (DDR1) gene was upregulated in these cells, and its suppression reduced motility and invasion without affecting AKT signaling. Clinically, higher ANKRD17 expression correlated with aggressive HCC progression. These findings suggest that ANKRD17 enhances metastatic progression in HCC by activating pro-metastatic and pro-survival pathways.
Keywords: cancer; cell biology; molecular biology.
© 2025 The Author(s).
Conflict of interest statement
The other authors declare no conflict of interest.
Figures

References
-
- Montella M., D'Arena G., Crispo A., Capunzo M., Nocerino F., Grimaldi M., Barbieri A., D'Ursi A.M., Tecce M.F., Amore A., et al. Role of Sex Hormones in the Development and Progression of Hepatitis B Virus-Associated Hepatocellular Carcinoma. Int. J. Endocrinol. 2015;2015 doi: 10.1155/2015/854530. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

