Metabolic Health Consequences of Switching to the Bictegravir/Emtricitabine/Tenofovir Alafenamide and Dolutegravir Plus Lamivudine Regimens in Virologically Suppressed People Living with HIV Aged > 40 Years: A Retrospective Real-World Study
- PMID: 40458197
- PMCID: PMC12129012
- DOI: 10.2147/IDR.S516775
Metabolic Health Consequences of Switching to the Bictegravir/Emtricitabine/Tenofovir Alafenamide and Dolutegravir Plus Lamivudine Regimens in Virologically Suppressed People Living with HIV Aged > 40 Years: A Retrospective Real-World Study
Abstract
Objective: Both B/F/TAF and DTG/3TC are recommended in treatment guidelines for switch therapy in PLWH. This study aimed to evaluate the safety and metabolic health consequences of two switched regimens in a real-world setting among virologically suppressed PLWH previously treated with EFV/TDF/3TC.
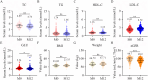
Methods: This retrospective real-world study in Hangzhou included 220 virologically suppressed PLWH who switched from EFV/TDF/3TC to DTG/3TC or B/F/TAF between January 1, 2020 and October 30, 2023. All participants were examined the changes in weight, BMI, GLU levels, lipid parameters (TC, LDL-C, HDL-C, and TG), and eGFR at post-12-month.
Results: The mean age of included participants was 50.8 years (SD: 11.3). After 12 months of switching, the HIV RNA level was below the limit of detection (< 20 copies/mL) among all participants. The switch to DTG/3TC or B/F/TAF therapy was associated with significant improvement in LDL-C, GLU levels, and eGFR values (all P < 0.05), while other metabolic indexes did not change significantly. Furthermore, there was a significant difference in the incidence of hyperglycemia (5.7% vs 19.35%; P = 0.004) between the B/F/TAF and DTG/3TC groups, but not included the mean changes of weight, BMI, lipid profiles, GLU levels, and eGFR and incidence of high TC and high TG. For the aged 40-59 years and aged ≥ 60 years PLWH, the differences in metabolic indicators were minimal between DTG/3TC and B/F/TAF groups post-12-month, with no significant differences between the arms in mean change from baseline in TC, TG, HDL-C, LDL-C, GLU, BMI, weight, and eGFR.
Conclusion: In this study, the B/F/TAF or DTG/3TC regimens are safe for virologically suppressed PLWH aged > 40 years. The transition to B/F/TAF demonstrated dual clinical benefits, significantly reducing hyperglycemia incidence while preserving renal function.
Keywords: antiretroviral therapy; metabolic indices; people living with HIV; virological suppression.
© 2025 Shi et al.
Conflict of interest statement
Regarding the publication of this paper, the authors declare that they have no conflicts of interest.
Figures


Similar articles
-
Safety and efficacy of lamivudine/dolutegravir vs. bictegravir/emtricitabine/tenofovir alafenamide in antiretroviral-naive adults with HIV-1 infection in Shanghai, China: a single-centre retrospective study.J Med Microbiol. 2025 Jan;74(1). doi: 10.1099/jmm.0.001949. J Med Microbiol. 2025. PMID: 39773780
-
Efficacy and safety profiles of dolutegravir plus lamivudine vs . bictegravir/emtricitabine/tenofovir alafenamide in therapy-naïve adults with HIV-1.Chin Med J (Engl). 2023 Nov 20;136(22):2677-2685. doi: 10.1097/CM9.0000000000002907. Epub 2023 Nov 2. Chin Med J (Engl). 2023. PMID: 37914678 Free PMC article.
-
Efficacy, Safety, and Tolerability of Switching From Bictegravir/Emtricitabine/Tenofovir Alafenamide to Dolutegravir/Lamivudine Among Adults With Virologically Suppressed HIV: The DYAD Study.Open Forum Infect Dis. 2024 Sep 26;11(10):ofae560. doi: 10.1093/ofid/ofae560. eCollection 2024 Oct. Open Forum Infect Dis. 2024. PMID: 39416993 Free PMC article. Clinical Trial.
-
An indirect comparison of 144-week efficacy, safety, and tolerability of dolutegravir plus lamivudine and second-generation integrase inhibitor-based, 3-drug, single-tablet regimens in therapy-naive people with HIV-1.AIDS Res Ther. 2023 Mar 22;20(1):17. doi: 10.1186/s12981-023-00507-1. AIDS Res Ther. 2023. PMID: 36949442 Free PMC article.
-
Comparison of the design and methodology of Phase 3 clinical trials of bictegravir/emtricitabine/tenofovir alafenamide (BIC/FTC/TAF) and dolutegravir-based dual therapy (DTG) in HIV: a systematic review of the literature.Expert Rev Anti Infect Ther. 2023 Jan;21(1):65-76. doi: 10.1080/14787210.2023.2149490. Epub 2022 Nov 27. Expert Rev Anti Infect Ther. 2023. PMID: 36399521
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

