Targeting cuproptosis for cancer therapy: Focus on the anti-tumor immune system
- PMID: 40458306
- PMCID: PMC12126744
- DOI: 10.1016/j.cpt.2024.07.005
Targeting cuproptosis for cancer therapy: Focus on the anti-tumor immune system
Abstract
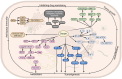
Copper (Cu) is an indispensable micronutrient that maintains signaling pathways and biological homeostasis in almost all cell types; however, its excess affects the tricarboxylic acid cycle, causes the accumulation of fatty acylated proteins, destabilization of iron-sulfur cluster proteins, and increases the levels of intracellular reactive oxygen species, leading to proteotoxic stress and cell death. Cuproptosis, a form of Cu-dependent cell death, differs from other types of regulated cell death (RCD) and was first reported in Science in 2022. Recently, the RCD pathways have been targeted in cancer therapy. However, the escape of apoptosis in tumor cells causes resistance to treatment and tumor recurrence. Therefore, there is an urgent need to study the alternative mechanisms of cancer cell mortality. Compared to normal patients, a significant increase in serum Cu ion levels has been observed in patients with tumors. Moreover, tumor cell proliferation, angiogenesis, and metastasis are associated with cuproptosis. Thus, exploring cancer signaling pathways related to cuproptosis will provide a new perspective for the development of anti-cancer drugs. Importantly, cuproptosis is closely associated with the modulation of anti-tumor immunity. The expression of cuproptosis-related genes (CRGs) is significantly correlated with immune cell infiltration and the immune checkpoint programmed cell death protein 1 (PD-1)/programmed death-ligand 1 (PD-L1). Based on these findings, a series of cuproptosis-related drugs have been used in tumor-targeted combination therapy or as immune synergists. Therefore, elucidating the role of cuproptosis per cancer stage and in the tumor immune microenvironment (TIME) is helpful in clarifying the potential value of Cu in the treatment of specific cancers. In this review, we summarize specific cancer signaling pathways related to cuproptosis and cancer treatment based on the regulation of Cu concentration. The combination of these two approaches may help researchers develop more therapies targeting cuproptosis-related pathways. Importantly, we focused on the effect of cuproptosis on the TIME and systematically discussed the role of CRGs in tumor immunity considering CRG-related anti-tumor immune signaling pathways, tumor prognosis scoring system, anti-tumor immunotherapy, and biological experiments and bioinformatics prediction models, to provide new ideas for the development of anticancer therapy targeting cuproptosis-related pathways.
Keywords: Copper homeostasis; Cuproptosis; Drug synergism; Immunotherapy.
© 2024 Published by Elsevier B.V. on behalf of Chinese Medical Association (CMA).
Figures


References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

