Epithelial-mesenchymal transition (EMT) and its role in acquired epidermal growth factor receptor-tyrosine kinase inhibitor (EGFR-TKI) chemoresistance in non-small cell lung cancer (NSCLC)
- PMID: 40458312
- PMCID: PMC12126741
- DOI: 10.1016/j.cpt.2024.07.001
Epithelial-mesenchymal transition (EMT) and its role in acquired epidermal growth factor receptor-tyrosine kinase inhibitor (EGFR-TKI) chemoresistance in non-small cell lung cancer (NSCLC)
Abstract
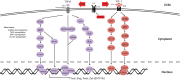
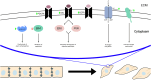
Epithelial-mesenchymal transition (EMT) is a biological process that involves the transformation of epithelial cells into cells with a mesenchymal phenotype, enhancing their migratory and invasive capabilities. EMT is crucial in embryonic development, tissue healing, and wound repair, and aids in forming diverse cell types and structures. However, aberrant EMT is involved in pathogenic processes, including fibrosis, cancer development, and progression. Recent studies show that EMT contributes to resistance to cancer treatment, including epidermal growth factor receptor-tyrosine kinase inhibitor (EGFR-TKI) therapy in non-small cell lung cancer (NSCLC). This review discusses the intricate relationship between EMT and acquired chemoresistance to EGFR-TKIs. It details evidence on how EGFR-TKIs might induce EMT and how EMT may cause EGFR-TKI resistance. Understanding these pathways is crucial for developing effective prognostic and therapeutic strategies to predict and combat acquired chemoresistance in NSCLC, advancing the field toward more targeted and personalized treatment approaches.
Keywords: Epidermal growth factor receptor; Epithelial–mesenchymal transition; Non-small cell lung cancer; Resistance; Tyrosine kinase inhibitors.
© 2024 The Author(s).
Figures


References
-
- Bubendorf L., Lantuejoul S., de Langen A.J., Thunnissen E. In: Dorfmüller Peter, Cavazza Alberto., editors. vol. 26. 2017. Nonsmall cell lung carcinoma: diagnostic difficulties in small biopsies and cytological specimens: Number 2 in the series “Pathology for the clinician”. (Eur Respir Rev). - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

