Tacca chantrieri André Rhizome Extract Alleviates Scopolamine-Induced Cognitive Impairment and Neuroinflammation in Rats
- PMID: 40458441
- PMCID: PMC12129611
- DOI: 10.1155/adpp/7334303
Tacca chantrieri André Rhizome Extract Alleviates Scopolamine-Induced Cognitive Impairment and Neuroinflammation in Rats
Abstract
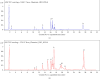
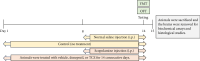
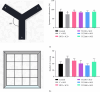
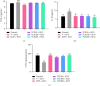
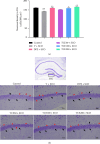
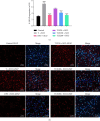
Tacca chantrieri André is a native plant from Northern Thailand with reported pharmacological effects, including antioxidant, anti-inflammatory, and neuroprotective properties. This study investigated the neuroinflammatory and cognitive-enhancing effects of Tacca chantrieri André rhizome extract (TCE) in a scopolamine-injected model, which mimics an Alzheimer's disease (AD) animal model. Animals were divided into six groups: (1) a control group, (2) a vehicle-treated group, (3) a donepezil-treated group (3 mg/kg BW) as a positive control, and (4-6) three TCE-treated groups receiving 50, 100, or 200 mg/kg BW once daily for 14 days. Starting on Day 8, animals received daily intraperitoneal injections of scopolamine (3 mg/kg BW) for 7 consecutive days to induce cognitive impairment. On day 14, behavioral tests were conducted, including the Y-maze and open field tests. On day 15, animals were euthanized, and their brains were collected for Nissl staining, immunofluorescence staining, and biochemical analyses using an ELISA kit. Our results demonstrated that TCE treatment attenuated scopolamine-induced memory deficits and neuroinflammation. Specifically, TCE administration reduced levels of proinflammatory cytokines, including tumor necrosis factor-α (TNF-α) and interleukin-1β (IL-1β), and decreased glial fibrillary acidic protein (GFAP) expression in the hippocampus. Additionally, TCE improved neuronal survival and enhanced serotonin levels, contributing to cognitive improvements. The qualitative analysis of TCE using LC-QTOF-MS identified various chemical constituents, including saponins, flavonoids, and phenolic compounds. These bioactive compounds contributed to the neuroprotective effects of TCE by modulating neuroinflammation and cognitive function. The neuroprotective effects of TCE suggested its potential as a therapeutic agent for memory impairment associated with AD.
Keywords: GFAP; Tacca chantrieri; memory; neuroinflammation; proinflammatory cytokine; scopolamine; serotonin.
Copyright © 2025 Thaneeya Hawiset et al. Advances in Pharmacological and Pharmaceutical Sciences published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
LinkOut - more resources
Full Text Sources
Miscellaneous
