Mapping the rare disease paediatric clinical trial availabilities in Europe
- PMID: 40458448
- PMCID: PMC12127375
- DOI: 10.3389/fped.2025.1523847
Mapping the rare disease paediatric clinical trial availabilities in Europe
Abstract
Introduction: The prevalence and complexity of rare diseases (RDs) require concerted efforts in research and clinical trial capabilities. This paper aims to map the clinical trial sites within the Collaborative Network for European Clinical Trials for Children (conect4children, c4c) consortium and the European Reference Networks for Rare Diseases (ERNs), assessing their potential overlap and opportunities for synergies to optimize the selection and preparedness of sites for paediatric RD clinical trials.
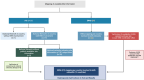
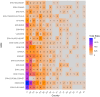
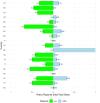
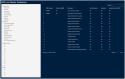
Method: A quantitative cross-mapping analysis was performed with publicly available data from ERN and c4c sites across 19 countries, complemented by information on paediatric site capabilities through interviews with network coordinators. Site analyses were done at country and setting levels. Heatmaps and an interactive matrix tool were developed using RStudio (v2023.12.0).
Results: The highest overlap between ERN and c4c networks is found in the Netherlands, Belgium, Sweden, Denmark, and the Czech Republic, indicating strong integration in these regions, while Nordic (Sweden and Denmark), Eastern, and Southern European countries show varying levels of overlap. The median proportion of regional sites to University sites is 0.05 (IQR 0.12) across ERNs and 0.25 (IQR 0.37) across c4c national networks. The matrix tool can identify overlap and its absence for both university and regional hospitals, enhancing the preparedness and reach of paediatric rare disease trials. ERN representatives confirm the heatmap and matrix tool's utility in improving site selection and fostering network cooperation.
Conclusion: Heatmap analyses reveal a significant but incomplete overlap of RD clinical trial sites between ERNs and c4c in parts of Europe, suggesting strong potential for cross-network collaboration to enhance paediatric RD trial recruitment and outcomes.
Keywords: drug development; network engagement; networks; paediatric; rare diseases c4c -ERN mapping clinical trial availabilities.
© 2025 Degraeuwe, Turner, Fernandes, Raes, Vande Walle and Schaefer.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures




References
-
- Orphanet: About rare diseases. Available at: https://www.orpha.net/consor/cgi-bin/Education_AboutRareDiseases.php?lng=EN (Accessed December 20, 2023).
-
- EUR-Lex - 32006R1901 - EN - EUR-Lex. Available at: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A32006R1901 (Accessed July 5, 2023).
LinkOut - more resources
Full Text Sources
Miscellaneous

