12-Month Outcomes of a Prospective Randomized Trial Investigating Effects of IVIG on Top of rATG Versus rATG Alone in Pre-Sensitized Kidney Transplant Recipients: The INHIBIT Study
- PMID: 40458542
- PMCID: PMC12127847
- DOI: 10.3389/ti.2025.14312
12-Month Outcomes of a Prospective Randomized Trial Investigating Effects of IVIG on Top of rATG Versus rATG Alone in Pre-Sensitized Kidney Transplant Recipients: The INHIBIT Study
Abstract
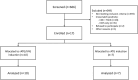
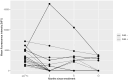
Intravenous immunoglobulins (IVIG) are commonly used in peri-transplant desensitization, but evidence supporting their efficacy is limited. We conducted a prospective, randomized single-center, open-label, Phase IIIb non-inferiority clinical pilot trial to compare the efficacy of IVIG (administered at a dose of 3 × 0.5 g/kg) versus no IVIG, in conjunction with rabbit anti-thymocyte globulin (5-7 mg/kg) induction, in pre-sensitized patients with donor-specific antibodies who had negative pre-transplantation Flow- and CDC-crossmatches, between July 2020 and November 2022. The primary endpoint was the rate of efficacy failure, defined as biopsy-proven rejection within 12-month post-transplant. Secondary endpoints included the incidence of rejection at protocol biopsies, evaluated by histology and biopsy-based transcripts diagnostics. Of the screened patients, 53 (72.6%) were excluded due to crossmatch positivity. Ten patients were randomized to the IVIG+, and 7 to the IVIG-arm. The trial was prematurely terminated due to futility at interim analysis. In the IVIG-arm, 3 patients (43%) experienced the primary endpoint compared to none in the IVIG+ arm (p = 0.026). MMDx identified one molecular ABMR in the IVIG+ and 2 in the IVIG-arm in 12-month protocol biopsies. There was one graft loss in the IVIG-arm. The results of this pilot study, although not definitive, do not support the use of IVIG-sparing regimens in HLA-incompatible kidney transplantation (NCT04302805). This study is registered on ClinicalTrials.gov under the identifier NCT04302805.
Keywords: HLA-incompatible transplantation; IVIG; desensitization; induction; kidney transplantation.
Copyright © 2025 Viklicky, Zahradka, Mares, Slatinska, Parikova, Petr, Roder, Jaklova, Osickova, Janousek and Hruba.
Conflict of interest statement
OV received speaker and/or consultancy honoraria from Astellas and Chiesi. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Schinstock CA, Mannon RB, Budde K, Chong AS, Haas M, Knechtle S, et al. Recommended Treatment for Antibody-Mediated Rejection after Kidney Transplantation: The 2019 Expert Consensus from the Transplantion Society Working Group. Transplantation (2020) 104:911–22. 10.1097/TP.0000000000003095 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials

