Distribution of intracellular calcium during flow-induced migration of RAW264.7 cells
- PMID: 40458545
- PMCID: PMC12128644
- DOI: 10.1016/j.mbm.2023.100012
Distribution of intracellular calcium during flow-induced migration of RAW264.7 cells
Abstract
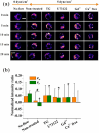
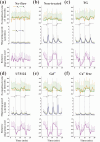
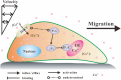
Cell migration is an important biological process regulated by mechanical stimulation, which leads to intracellular calcium response. Cell migration are dependent on the distribution and dynamic changes of intracellular calcium concentration. However, the temporal relation among mechanical stimulation, cell migration, and intracellular calcium distribution remains unclear. In this study, unidirectional flow and oscillatory flow were applied on osteoclast precursor RAW264.7 cells. The parameters of cell migration under fluid flow and intracellular calcium distribution along the migration or flow direction were calculated. Experimental results suggest the cells to adjust the [Ca2+]i distribution in the migration direction is independent of flow application or the reverse of flow direction, but the [Ca2+]i distribution in the flow direction is determined by the [Ca2+]i distribution-adjusting ability of cells and flow stimulation. Blocking calcium signaling pathways, namely, mechanosensitive cation-selective channels, phospholipase C, and endoplasmic reticulum, and removing extracellular calcium inhibited cell migration along the flow direction and the gradient distribution of intracellular calcium. This study provided insights into the mechanism of flow-induced cell migration and quantitative data for the recruitment of osteoclast precursors targeting the location of bone resorption.
Keywords: Calcium signaling; Cell migration; Fluid shear stress; Intracellular calcium distribution; Osteoclast precursor.
© 2023 The Author(s).
Conflict of interest statement
Shurong Wang, Qing Sun, Yang Zhao, Bo Huo declare that they have no conflicts of interest.
Figures



References
LinkOut - more resources
Full Text Sources
Miscellaneous

