Role of body anthropometry in severe asthmatic patients: Evidences from the Severe Asthma Network in Italy (SANI) registry
- PMID: 40458738
- PMCID: PMC12127536
- DOI: 10.1016/j.waojou.2025.101056
Role of body anthropometry in severe asthmatic patients: Evidences from the Severe Asthma Network in Italy (SANI) registry
Abstract
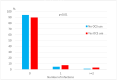
Asthma and obesity are both chronic diseases. Obesity is a common comorbidity and a risk factor of severe asthma, associated with increased asthma exacerbation risk, poorer asthma control and reduced quality of life. However, the responsible mechanisms are poorly understood. The aim of this study was to detect parameters associated with obesity in patients with severe asthma in order to check different pattern of inflammation in obese asthmatics. Baseline data from the Severe Asthma Network in Italy (SANI) registry were analysed in 1922 patients with severe asthma. Demographic, clinical and functional features were compared, according to body mass index (BMI). The prevalence of overweight and obesity among severe asthma patients was 34,8 and 20,3, respectively. Females were more prevalent in the obese cluster (p < 0.001). Asthma onset age in overweight and obese patients was higher than in normal population (p < 0.001). Obese subjects reported less frequently chronic rhinosinusitis with nasal polyposis (CRSwNP) and more frequently impaired sleep quality, cardiovascular disease, and type-2 diabetes (p < 0.001). Severe asthma patients with obesity had lower predicted FVC values (89.0 ± 19.2 vs 93.5 ± 20.2; p 0.002) and higher FEV1/FVC ratio (69.9 ± 11.5 vs 66.9 ± 12.4; p < 0.001) than patients without obesity. Obese asthmatics had lower blood eosinophilic count, and fractional exhaled nitric oxide (FeNO) levels than non-obese asthmatics. Asthma control test (ACT) was significantly poorer in obese patients (17, IQR 12-21) than other subgroups. Regarding treatment, overweight and obese patients were more likely to receive a GINA-Step 5 therapy (p 0.023), with more than 20 of obese asthmatics having frequent exacerbations requiring oral corticosteroid (OCS). Patients with severe asthma and obesity presented different characteristics that support the existence of distinct asthma phenotype in obese patients.
Trial registration: Trial registry: ClinicalTrials.gov. ID: NCT06625216. Retrospectively registered October 3, 2024.
Keywords: Asthma; BMI; Lung function; Obesity.
© 2025 The Authors.
Conflict of interest statement
ER reports grants or contracts and payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Menarini International. MM reports payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events from AstraZeneca and payment for expert testimony from GSK. FB reports financial grants from AstraZeneca, Chiesi Farmaceutici S.p.A and Insmed Inc. outside the submitted work; Consulting fees from Menarini and Zambon outside the submitted work; Speaker fees from AstraZeneca, Chiesi Farmaceutici S.p.A, Glaxo Smith Kline, Guidotti, Grifols, Insmed Inc., Menarini, Novartis AG, Sanofi-Genzyme, Viatris Inc., Vertex Pharmaceuticals and Zambon outside the submitted work. PP reports grants for educational events from AstraZeneca, Chiesi Farmaceutici, Glaxo Smith Kline, Guidotti and Sanofi outside the submitted work; grants for participation to Advisory Board from Chiesi Farmaceutici, Glaxo Smith Kline, and Sanofi outside the submitted work. EH reports personal fees for consultancy, for lectures, presentations, speakers bureaus, manuscript writing or educational events, and for participation on a Data Safety Monitoring Board or Advisory Board from: GSK, Sanofi, Regeneron, Novartis, AstraZeneca, Stallergenes-Greer, Chiesi, Almirall, Bosch, and Lofarma. LB reports fees for lessons and consultancy, payment or honoraria for lectures and presentations as well as support for attending meetings and/or travel from AstraZeneca, GSK, Novartis, and Sanofi; grants for participation on a Data Safety Monitoring Board or Advisory Board from AstraZeneca, GSK, and Sanofi; participation on the National Board of directors of SIAAIC, as President of ATI Eosinophils, and chair of MASK-air Italy. GWC reports research grants, honoraria for lectures and Advisory Board fees from A. Menarini, Allergy Therapeutics, AstraZeneca, Chiesi Farmaceutici, Faes, Firma, Glaxo Smith Kline, Guidotti-Malesci, Hal Allergy, Innovacaremd, Novartis, OmPharma, RedMaple, Sanofi-Aventis, Sanofi-Genzyme, Stallergenes-Greer, Uriach Pharma, ThermoFisher, Valeas outside the submitted work and fees for lectures or advisory board participation from Menarini, AstraZeneca, CellTrion, Chiesi, Faes Farma, Firma, Genentech, Guidotti-Malesci, GSK, HAL Allergy, Innovacaremd, Novartis, OM-Pharma, Red Maple, Sanofi-Aventis, Sanofi-Genzyme, Stallergenes-Greer and Uriach Pharma. GS reports financial grants and fees for Advisory Board from AstraZeneca, GSK, Novartis, and Sanofi. MC reports financial grants from AstraZeneca, GSK, and Sanofi; consulting fees from AstraZeneca; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events from AstraZeneca and Sanofi. All other authors do not have any conflict of interest to declare.
Figures
References
-
- Global Initiative for Asthma Global strategy for asthma management and prevention. 2023. https://www.ginaasthma.org Available from: updated.
-
- Chung K.F., Wenzel S.E., Brozek J.L., et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J. 2014;43:343–373. Eur Respir J. 2022 Jun 9;59(6):1362020. doi: 10.1183/13993003.62020-2013. Erratum for: Eur Respir J. 2014 Feb;43(2):343-73. PMID: 35680153. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical

