Study protocol and statistical plan for the ICRAKI trial: Intermittent haemodialysis versus continuous renal replacement therapy for severe acute kidney injury in critically ill patients
- PMID: 40458742
- PMCID: PMC12127651
- DOI: 10.1016/j.ccrj.2025.100107
Study protocol and statistical plan for the ICRAKI trial: Intermittent haemodialysis versus continuous renal replacement therapy for severe acute kidney injury in critically ill patients
Abstract
Background: The effect of intermittent haemodialysis (IHD) vs continuous renal replacement therapy (CRRT) on mortality and/or renal function recovery in adults with acute kidney injury (AKI) and a recognised indication for renal replacement therapy (RRT) remains controversial.
Objective: To summarise the protocol and statistical analysis plan for the ICRAKI trial.
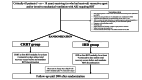
Design settings and participants: ICRAKI is a non-inferiority multicentre randomised controlled trial comparing IHD and CRRT. We will include 1000 patients with AKI receiving (or who have received) invasive mechanical ventilation and/or catecholamine infusion and who have at least one recognised criterion for initiating RRT.
Intervention: The study compares IHD with CRRT.
Main outcomes measures: The primary endpoint is the proportion of patients who will meet one or more criteria for a major adverse kidney event (composite of death, RRT dependence and/or more than a 25 % increase in serum creatinine from baseline value) 90 days after randomisation. Secondary endpoints are time to death; mortality at day (D)28, D60 and D90; number of patients with RRT dependency at D28, D60 and D90; number of patients with more than a 25 % increase in serum creatinine from baseline value at D28, D60 and D90; intensive care unit (ICU) and hospital length of stay; time until cessation of RRT; catecholamine-free days, ventilator-free days and RRT-free days through day 28; estimated glomerular filtration rate at hospital discharge; the number of episodes of adverse events.
Conclusion: The ICRAKI trial will inform the choice of RRT modalities in critically ill patients with severe AKI. More than 300 patients were already included.
Trial registration: ClinicalTrials.gov: NCT06032884. Date of registration, 2023-09-04.
Keywords: Acute kidney injury; Critical care; Renal replacement therapy; Treatment outcome.
© 2025 The Authors.
Conflict of interest statement
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: GAUDRY STEPHANE reports financial support was provided by the French Government Ministry of Social Affairs Health and Women’s Rights. If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
References
-
- Hoste E.A.J., Bagshaw S.M., Bellomo R., Cely C.M., Colman R., Cruz D.N., et al. Epidemiology of acute kidney injury in critically ill patients: the multinational AKI-EPI study. Intensive Care Med. août. 2015;41(8):1411–1423. - PubMed
-
- Ronco C., Bellomo R., Kellum J.A. Acute kidney injury. Lancet Lond Engl. 2019;394(10212):1949–1964. 23. - PubMed
-
- Bagshaw S.M., Darmon M., Ostermann M., Finkelstein F.O., Wald R., Tolwani A.J., et al. Current state of the art for renal replacement therapy in critically ill patients with acute kidney injury. Intensive Care Med. juin. 2017;43(6):841–854. - PubMed
-
- Truche A.S., Darmon M., Bailly S., Clec’h C., Dupuis C., Misset B., et al. Continuous renal replacement therapy versus intermittent hemodialysis in intensive care patients: impact on mortality and renal recovery. Intensive Care Med. sept. 2016;42(9):1408–1417. - PubMed
-
- Schneider A.G., Bellomo R., Bagshaw S.M., Glassford N.J., Lo S., Jun M., et al. Choice of renal replacement therapy modality and dialysis dependence after acute kidney injury: a systematic review and meta-analysis. Intensive Care Med. juin. 2013;39(6):987–997. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical