Xiaoyao San ameliorates maternal inflammation-induced neurobehavioral deficits by modulating the microbiota-gut-brain axis in offspring
- PMID: 40458794
- PMCID: PMC12127538
- DOI: 10.3389/fphar.2025.1563496
Xiaoyao San ameliorates maternal inflammation-induced neurobehavioral deficits by modulating the microbiota-gut-brain axis in offspring
Abstract
Background: XiaoYao San (XYS), a classical Traditional Chinese Medicine (TCM), has demonstrated efficacy in alleviating stress-related neuropsychiatric disorders. However, its therapeutic potential against maternal immune activation (MIA)-induced neurobehavioral impairments remains unexplored. This study aims to investigate the neuroprotective effects of XYS on MIA-related behavioral dysfunctions and elucidate its underlying mechanisms.
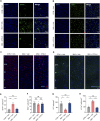
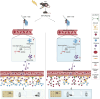
Results: Using a poly (I:C)-induced MIA mouse model, we demonstrated that XYS effectively ameliorates autism spectrum disorder (ASD) related behavioral phenotypes. Mechanistic investigations revealed that XYS exerts its therapeutic effects through: (1) Attenuation of core behavioral deficits including enhanced social interaction and reduced repetitive behaviors; (2) Downregulation of intestinal amino acid transporters; (3) Restoration of cerebral glutamate-GABA balance via modulation of glutamine pathway; (4) Structural remodeling of gut microbiota with specific enrichment of Bacteroides spp. Notably, B. uniformis was identified as a key microbial mediator capable of recapitulating XYS-mediated neurophysiological improvements through metabolic regulation.
Conclusion: This study elucidates XYS as a multi-target therapeutic agent that coordinately modulates gut microbial ecosystems, amino acid homeostasis, and neurotransmitter homeostasis. The findings provide novel insights into the gut-brain axis mechanisms of TCM formulations, offering a scientific foundation for developing microbiota-based intervention strategies for neurodevelopmental disorders.
Keywords: Xiaoyao san; amino acid transporter; gut microbiota; maternal immune activation (MIA); neurodevelopmental disorders.
Copyright © 2025 Lin, Zhu, Zhang, Shi, Zhong, Xia, Ding and Yang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures






Similar articles
-
Xiaoyao San ameliorates high-fat diet-induced anxiety and depression via regulating gut microbiota in mice.Biomed Pharmacother. 2022 Dec;156:113902. doi: 10.1016/j.biopha.2022.113902. Epub 2022 Oct 21. Biomed Pharmacother. 2022. PMID: 36279723
-
Xiaoyaosan against depression through suppressing LPS mediated TLR4/NLRP3 signaling pathway in "microbiota-gut-brain" axis.J Ethnopharmacol. 2024 Dec 5;335:118683. doi: 10.1016/j.jep.2024.118683. Epub 2024 Aug 8. J Ethnopharmacol. 2024. PMID: 39121928
-
Studies on the potential link between antidepressant effect of Xiaoyao San and its pharmacological activity of hepatoprotection based on multi-platform metabolomics.J Ethnopharmacol. 2020 Mar 1;249:112432. doi: 10.1016/j.jep.2019.112432. Epub 2019 Nov 29. J Ethnopharmacol. 2020. PMID: 31790818
-
Modified xiaoyao san combined with chemotherapy for breast cancer: A systematic review and meta-analysis of randomized controlled trials.Front Oncol. 2023 Mar 22;13:1050337. doi: 10.3389/fonc.2023.1050337. eCollection 2023. Front Oncol. 2023. PMID: 37035186 Free PMC article.
-
IUPHAR review: Targeted therapies of signaling pathways based on the gut microbiome in autism spectrum disorders: Mechanistic and therapeutic applications.Pharmacol Res. 2025 Jan;211:107559. doi: 10.1016/j.phrs.2024.107559. Epub 2024 Dec 28. Pharmacol Res. 2025. PMID: 39733842 Review.
References
-
- Andersen J. V., Christensen S. K., Westi E. W., Diaz-delCastillo M., Tanila H., Schousboe A., et al. (2021a). Deficient astrocyte metabolism impairs glutamine synthesis and neurotransmitter homeostasis in a mouse model of Alzheimer's disease. Neurobiol. Dis. 148, 105198. 10.1016/j.nbd.2020.105198 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

