Genetic Profiling Reveals the Distinctions Among MTX-Associated DLBCL, EBV-Positive Mucocutaneous Ulcer, and EBV + DLBCL
- PMID: 40458922
- PMCID: PMC12317404
- DOI: 10.1111/cas.70111
Genetic Profiling Reveals the Distinctions Among MTX-Associated DLBCL, EBV-Positive Mucocutaneous Ulcer, and EBV + DLBCL
Abstract
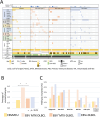
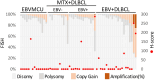
The WHO recently changed the outline of immunodeficiency/dysregulation (IDD)-associated lymphoproliferative disorders (LPDs)/lymphomas from underlying IDD settings to an overarching framework and accommodates commonalities in histology, the involvement of various oncogenic viruses, and specific clinical/therapeutic consequences. A mutational analysis has been performed on post-transplantation and HIV-positive lymphomas, but not on other iatrogenic immunodeficiency (OII)-associated LPDs mainly caused by methotrexate (MTX) to treat rheumatoid arthritis. We herein conducted next-generation sequencing (NGS) to examine the genetic spectrum along with a fluorescence in situ hybridization analysis of 9p24.1 and PD-L1 expression in 37 MTX-associated diffuse large B-cell lymphoma (DLBCL) cases, 17 Epstein-Barr virus (EBV)-positive mucocutaneous ulcer (EBVMCU) cases, and 26 EBV-positive DLBCL (EBV + DLBCL) cases. Targeted NGS identified 177 mutations. The mutation frequency was significantly higher in EBV- MTX-DLBCL than in EBV-positive LPDs/lymphomas (EBVMCU, EBV+ MTX-DLBCL, and EBV + DLBCL). Regrowth or resistance to spontaneous regression after MTX withdrawal was more likely in EBV- MTX-DLBCL than in EBV+ MTX-DLBCL. Therefore, accumulated gene mutations, sustained by the restored immune status in EBV- MTX-DLBCL, may affect clinical outcomes after MTX discontinuation. Several unique genetic findings were obtained for each category. Fewer TET2/DNMT3A and CD58 mutations in OII-LPD/lymphomas (EBVMCU and MTX-DLBCL) than in EBV + DLBCL indicate that clonal hematopoiesis and an immune evasion-related background contributed less to lymphomagenesis in OII-LPDs. MYD88L265P/CD79BY196 mutations were only detected in EBV- MTX-DLBCL. SOCS1 mutations were significantly more frequent in EBV-positive LPD/lymphoma categories, irrespective of the immune status, than in EBV- MTX-DLBCL. These results reveal distinct genetic features among MTX-DLBCL (EBV+/-), EBVMCU, and EBV + DLBCL.
© 2025 The Author(s). Cancer Science published by John Wiley & Sons Australia, Ltd on behalf of Japanese Cancer Association.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




References
-
- Swerdlow S. H., Campo E., Harris N. L., et al., Post‐Transplant Lymphoproliferative Disorders. WHO Classification of Tumours of Hematopoietic and Lymphoid Tissue. Revised 4th ed (IARC, 2017).
-
- Gru A. A. and O'Malley D. P., “Autoimmune and Medication‐Induced Lymphadenopathies,” Seminars in Diagnostic Pathology 35, no. 1 (2018): 34–43. - PubMed
-
- Hasserjian R. P., Chen S., Perkins S. L., et al., “Immunomodulator Agent‐Related Lymphoproliferative Disorders,” Modern Pathology 22, no. 12 (2009): 1532–1540. - PubMed
-
- Hoshida Y., Tsujii A., Ohshima S., et al., “Effect of Recent Antirheumatic Drug on Features of Rheumatoid Arthritis‐Associated Lymphoproliferative Disorders,” Arthritis & Rhematology 76, no. 6 (2024): 869–881. - PubMed
-
- de Jong D., Chan J. K. C., Coupland S. E., et al., Lymphoid Proliferations and Lymphomas Associated With Immune Deficiency and Dysregulation. WHO Classification of Tumours Editorial Board. Haematolymphoid Tumours. 5th ed (IARC, 2024). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

